 A critical part of any coatings formulation is the contribution that the resin has to the ultimate performance attributes of the coating. In this article, we will define some key resin parameters and how they influence resin selection in light of the desired coating properties. Some of the key definitions of resin properties we will define include thermoplastic, thermoset, glass transition temperature, tensile strength, modulus, elongation, functionality, film formation temperature, molecular weight, weight average molecular weight, number average molecular weight and molecular weight distribution.
A critical part of any coatings formulation is the contribution that the resin has to the ultimate performance attributes of the coating. In this article, we will define some key resin parameters and how they influence resin selection in light of the desired coating properties. Some of the key definitions of resin properties we will define include thermoplastic, thermoset, glass transition temperature, tensile strength, modulus, elongation, functionality, film formation temperature, molecular weight, weight average molecular weight, number average molecular weight and molecular weight distribution.
Film Formation – Thermoset and Thermoplastic
Simply stated, a thermoplastic resin softens with heat, whereas a thermoset resin has functional groups and is thus capable of crosslinking with a reactant or self-crosslinking. Thermosetting resins react and harden in the presence of heat, moisture and/or oxygen. Film formation for a liquid paint using a thermoplastic resin takes place by water or solvent evaporation, whereas film formation for liquid coatings containing thermosetting resins occurs in two stages. In the first stage, solvent and/or water is evaporated and in the second stage, the resin self cross-links or reacts in the presence of an external cross-linker or reactant. For most applications requiring high levels of chemical resistance and toughness, thermosetting resins are normally the technology of choice. Unlike a thermosetting coating, when a thermoplastic coating is heated, it softens appreciably, as it is thermo-plastic.
Resin Characterization – Glass Transition Temperature, Molecular Weight and Molecular Weight Distribution
Two other considerations that have a pronounced influence on coating performance are glass transition temperature and molecular weight, including molecular weight distribution. The vast majority of synthetic polymers used in coatings are amorphous rather than crystalline, so they do not have a distinct melting point. Amorphous polymers have one or more temperatures in which there is a change in the rate of increase of specific volume with increasing temperature. Properly defined, the glass transition temperature is the temperature at which there is an increase in the thermal expansion coefficient (change from a glassy state where the resin is hard and brittle to a more rubbery state). The Tg can be measured using dynamics mechanical analysis (DMA) or differential scanning calorimetry (DSC).
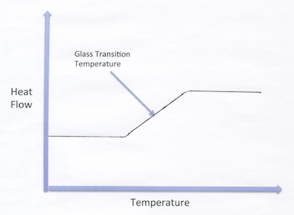

Tg is a very important consideration in the selection of the proper resin for a given application. For example, a resin with a Tg significantly above room temperature may not properly coalesce to form a good film at room temperature. Conversely, for a thermoplastic ambient cure system, a resin with a Tg below room temperature will form a soft film that is not very mar or scratch resistant. At the Tg, the free volume and thus the mobility of the resin backbone increases. So for thermosetting resins, this also enables greater availability of resin functionalities to react or crosslink to form a film. As you can see from the above chart, a number of fundamental properties are dependent on the Tg of the resin within a given polymer type. Another important characteristic that influences the performance of a resin in a coatings formulation is the molecular weight (MW) and the molecular weight distribution. Synthetic polymers (EU) contain a mixture of polymers with different molecular weights.

There is a mixture of MW’s in a synthetic polymer. Thus the MW’s can be defined only by using a statistical distribution. The weight average molecular weight, or Mw, is defined as the summation of the products of the weight of each polymer species at a given molecular weight divided by the total molecular mass. The number average molecular weight or Mn is the molecular weight of the summation of the products of the number of molecules at each molecular weight divided by the sum of the number of molecules in a given sample. The ratio of Mw/Mn is called the molecular weight distribution or also polydispersity.
The molecular weight, as well as the molecular weight distribution, can have a profound effect on coating performance. For example, for coatings made with resin solutions, normally the higher the molecular weight, the higher the viscosity. This is a most critical consideration when formulating high solids coatings, since at the same viscosity, a higher molecular weight resin will require more solvent, resulting in a higher VOC than the same resin composition supplied at a lower molecular weight. Higher molecular weights, especially for thermoplastic polymers, normally result in higher tensile strength (toughness). For higher solids coatings, a narrow molecular weight distribution is desirable as lower viscosity results. Viscosity dependence on molecular weight is much less of an issue in water born coatings, because most of these coatings are comprised of polymeric particles (EU) (emulsion, dispersion or micro-emulsion) rather than solutions.
Mechanical Properties – Tensile Strength, Modulus, Elongation and Yield Strength
Most coatings formulators were trained as chemists and, unlike engineers, have had little or no exposure to terms such as tensile strength, modulus and elongation. However, mechanical properties of a resin system have a profound impact on the initial and longer-term performance of the coatings these resins are used in. Following is a brief description of these terms and how they impact coatings performance. Mechanical properties are determined by subjecting the sample to a stress-strain test. These properties are determined by subjecting a sample to a stress (force/unit area) strain test (% elongation) as Figure 1 illustrates.
Elongation-at-break is usually expressed as the length at break (A) after stretching (amount of strain) divided by the original length. Tensile strength or tensile-at-break is a measure of the stress when the sample breaks (B). The slope of the stress-strain plot (C) is the ratio of stress/strain or the modulus. Another way of describing modulus is the ability of a sample of a material to resist deformation. The area under the curve is described as the work-to-break. As the sample is subjected to additional strain, the stress required for additional elongation decreases. The maximum stress at this point is called the yield point (D). The yield point is normally described in two ways, either yield strength (E) or elongation-at-yield.
Stress-strain plots are impacted by temperature and the rate of pull (time). Accordingly, the conditions under which these plots are determined is important when comparing plots. The determination of the mechanical properties of the resins chosen beforehand for use in a coating formulation can be an extremely helpful parameter.
In summary, having a full understanding of the resin characteristics and mechanical properties of a resin type can streamline the formulation process and provide the insight needed to formulate a successful product. If requested, many suppliers will provide the detail necessary to ensure that the resin selected is suitable to meet your performance specifications. There are numerous resin suppliers listed in UL’s Prospector website with a wide variety of resin technologies to meet your requirements.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.





Is there a chart comparing the physical performance properties of coatings resin. ie. when to use which resin type – alkyd, vinyl acrylic, styrene acrylic, polyester, polyurethane, etc.
Which one is best for outdoor, indoor, UV, corrosion, scratch resistance, adhesion, chemical resistance etc?
Hello Chris,
Thank you for your question, the answer to which is also dependent on whether you are comparing a crosslinked thermoset system to an ambient cure system and if crosslinked, what the crosslinker is. Feel free to contact me at [email protected] for a more detailed response/explanation.
Thank you,
Ron Lewarchik