 Exterior weathering can have a dramatic effect on the aesthetic, functional and physical properties of coatings that can include chalking, film erosion, cracking, color change, etching, blisters, peeling, spotting, and loss of hardness, flexibility (increase in glass transition temperature, or Tg), gloss, and adhesion. Multiple formulation issues influence the performance of coatings in a given exterior environment and include:
Exterior weathering can have a dramatic effect on the aesthetic, functional and physical properties of coatings that can include chalking, film erosion, cracking, color change, etching, blisters, peeling, spotting, and loss of hardness, flexibility (increase in glass transition temperature, or Tg), gloss, and adhesion. Multiple formulation issues influence the performance of coatings in a given exterior environment and include:
- Resin Type
- Crosslinker Type
- Pigment/color
- Pigment type
- Pigment to binder ratio
- Presence of Catalyst
- Additive selection
Need help researching antioxidants and UV absorbers for coatings?
Prospector can help speed along your research with technical datasheets and access to global suppliers for thousands of materials.
Create your free account today!
What impacts exterior coating weathering?
Some of these factors will be covered in more detail than others to the degree they influence weathering. The major issues impacting exterior weathering include: photooxidation (presence of oxygen and light) and hydrolysis due to the effects of moisture, heat and light. The former can be mitigated to a degree with the proper use of UV absorbers to reduce exposure of the polymer matrix to UV light, antioxidants and hindered amine light stabilizers (HALS) to reduce the effects of associated oxidative degradation.
Both photooxidation and hydrolysis are exacerbated by an increase in temperature as both are thermally activated. Environments high in airborne moist salt and/or acid rain (high sulfate, nitrate) and ozone accelerate the hydrolysis and degradation of resin systems and accelerate color change due to acid attack of pigments.
By far the major process that influences film degradation of polymeric coatings is photooxidation. Oxidative degradation proceeds by hydrogen abstraction from the polymer through an autocatalytic process. Accordingly, to achieve excellent weathering, avoid or at least minimize functional groups in the polymer that are more vulnerable to hydrogen abstraction. Following is a general order of functional group resistance to oxidative degradation of activated methylene groups (- CH2–) between double bonds or adjacent to amine groups being the worst:

Accordingly, in general fluoropolymers and siloxanes are more durable than polyesters or urethanes followed by resin systems high in aromatic content, and amine groups being the least durable. The later types include aromatic epoxies.
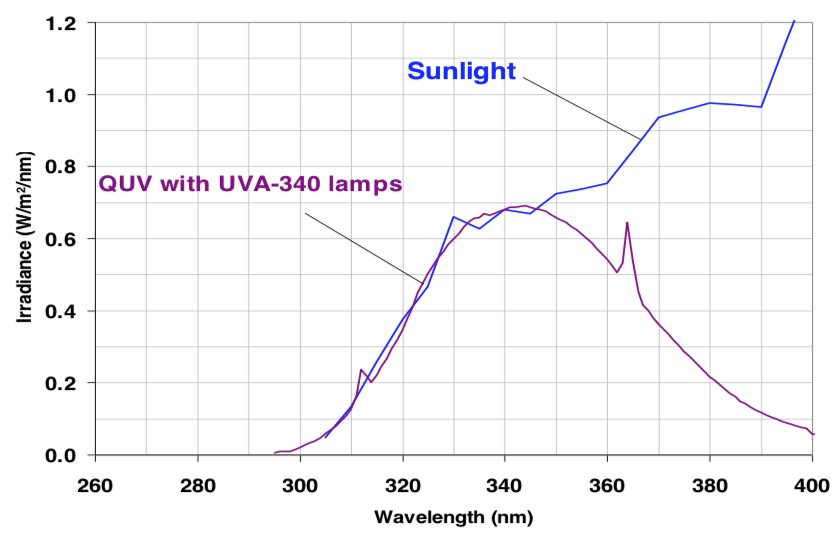
Characteristics of UV Stabilizers include absorption and quenching. UV absorbers act by absorbing radiation in the wavelength region where the polymer system absorbs thus acting to shield the resin from degradation. Ideally UV Stabilizers should have a high absorption in the UV region from 295 to 380nm to provide protection for the polymer from degradation. The most effective UV stabilizers are also more permanent thus ensuring longer life once incorporated into a paint system.
UV stabilizers convert the absorbed UV energy into heat, such as that with 2 – hydroxy benzophenone:
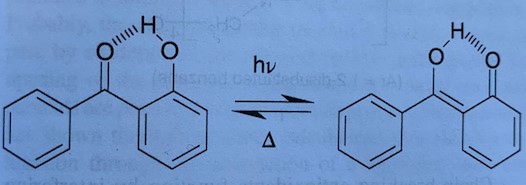
Antioxidants are classified into two groups of preventative (peroxide decomposers and chain breaking antioxidants). Peroxide decomposers include sulfides and phosphites. Chain breaking antioxidants disrupt the chain propagation step of autoxidation. Organic materials react with molecular oxygen in a process called “autoxidation“. Autoxidation is initiated by heat, light (primarily in the UV region), mechanical stress, catalyst residues, or reaction with impurities to form alkyl radicals. The free radical can, in turn, react and result in the degradation of the polymer such as depicted below:
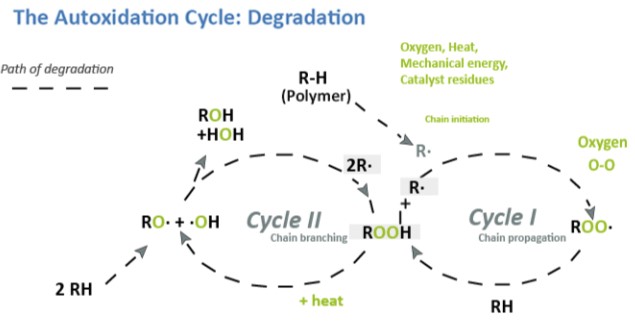
Hindered Amine Light Stabilizers (HALS) function both as chain breaking antioxidants as well as complexing agents for transition metals. For coatings that provide excellent durability, the rate of Hydrolysis is normally much lower than that of photooxidation.
The rate of hydrolysis for functional groups is esters>carbonates>ureas>urethanes>ethers.
For crosslinked products, melamines hydrolyze at a faster rate than that of aliphatic urethanes.
As most systems used in exterior applications contain pigment (including basecoat/clearcoat systems used in exterior automotive topcoats); pigment selection, color as well as pigment volume concentration (PVC) all contribute to the durability of the paint system. PVC selection is somewhat dictated by gloss level, color requirement and film thickness necessary for acceptable hide (color uniformity over the substrate).
In paint systems dependent on protection provided by pigment for light stability, durability is more dependent on relatively small variations in PVC. The relationship between color and weathering can be very complicated. For example, darker colors tend to absorb more radiant energy and thus the heat absorption coefficient for darker colors not using solar reflective pigments is higher, contributing to higher temperatures of the coating exposed to exterior radiant energy:
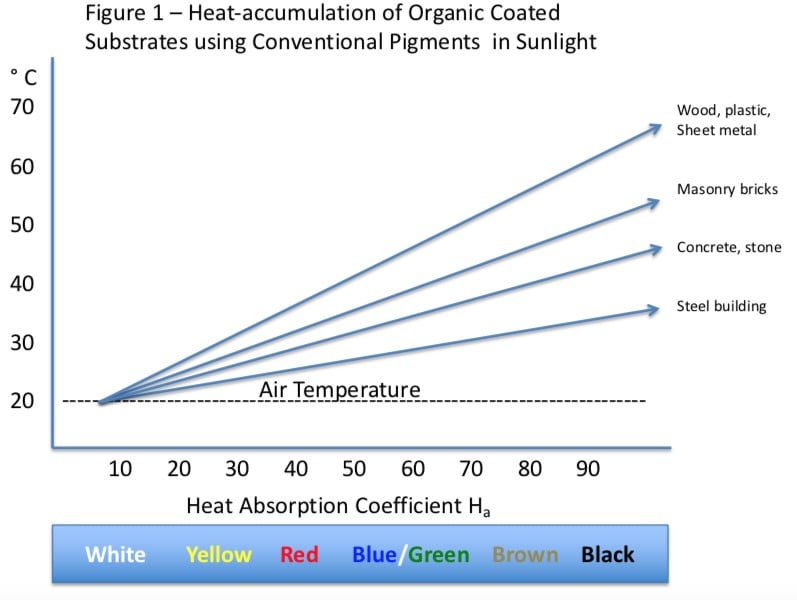
Higher temperatures contribute to higher degradation rates, however darker colors (brown/black) absorb more UV/Visible light energy and thus help protect the polymer system from degradation. Accordingly the use of a resin system prone to oxidative degradation at higher temperatures will provide poor weathering especially in dark colors.
Pigment selection within a class of colors can have a tremendous effect on the durability within a class of polymers. Pigments used for color and hiding can be divided into two general classes including inorganic and organic.
Inorganic pigments as a class are more resistant to degradation and chemicals than are organics. Some of the durable inorganic pigments include acid resistant aluminum flake, micaceous iron oxide, yellow, brown and red iron oxides.
The most durable inorganic pigments are Ceramic pigments. Ceramic pigments are mixed metal oxides. As these pigments are fully oxidized they are very resistant to chemicals and oxidation. As many bright colors require organic pigments, such pigments are a necessity. Many organic pigments can provide exceptional resistance to exterior degradation and are used extensively in automotive basecoats.
How to evaluate coating weathering
The best way to evaluate weathering is by natural exposure in the color, environment, gloss level and exposure angle the coating will be used in. As that is not practical for the introduction of new coatings, accelerated weathering is a necessity.
South Florida weathering is normally the most accepted means to determine accelerated natural weathering of a coating. For example: 5 degrees horizontal south facing for automotive applications or 45 or 90 degrees facing south or north respectively for architectural applications.
Marine environments are also commonly used for paint systems to evaluate corrosion protection or resistance to biological growth. Although South Florida weathering provides a good indication of the projected durability, there is always a desire to further reduce the time required to predict the durability of a coating to an environment high in UV, moisture, and high temperature.
A few of the other commonly used methods to determine accelerated weathering include ASTM D 4587 (QUV weathering) and ASTM G155/ASTM D7869 (Xenon Arc). These accelerated weathering devices provide a combination of cycles of intense UV light, high temperature and high humidity. There are a number of articles detailing the correlation or lack thereof with natural weathering including new instruments and processes that profess to provide a better correlation to natural weathering.
Sources and further reading:
- Prospector Search Engine
- UV Absorbers
- antioxidants
- ceramic pigments
- mixed metal oxide
- HALS
- iron oxide
- UV stabilizer
- Organic Coatings, Science and Technology, Frank N. Jones et.al., Wiley & Sons, 2017
- Exterior Durability of Organic Coatings, Eric V. Schmid, FMJ International, 1988
- The Right Choice, Jim Regan, Q Lab
- Prospector Knowledge Center: Resin Fundamentals and Their Effect of Coatings Performance, R. Lewarchik
- Prospector Knowledge Center: Effect of PVC, R. Lewarchik
- Prospector: Architectural Coatings that Reduce Heating and Cooling Costs, R. Lewarchik
- SpecialChem: Antioxidants to Prevent Polymer Oxidation
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Hello Mr.Lawarchik,
Please advise on the following :
“Higher temperatures contribute to higher degradation rates, however darker colors (brown/black) absorb more UV/Visible light energy and thus help protect the polymer system from degradation. Accordingly the use of a resin system prone to oxidative degradation at higher temperatures will provide poor weathering especially in dark colors.”
If dark colors absorb UV , thus protecting the Polymer system, then resin system prone to oxidative degradation should have better weathering in dark colors as compared to light colors. Please advise.
Hello Kuldeep,
Thank you for your comment. Darker colors and specifically black provide additional UV/Visible light opacity versus more transparent colors. Coatings with a high level of Rutile titanium dioxide also provide high UV/Vis opacity (minimal light absorption). Minimizing the absorption of UV/Vis Light helps to protect the resin resin system from UV/Vis induced degradation. However, darker colors using nonIR reflective pigments absorb more IR energy and thus darker coatings will experience higher temperatures as they absorb IR energy. Higher temperatures accelerate the degradation of certain polymers due to higher oxidation rates. Please see the graphs and mecanisms in the article for more detail.
Thank you,
Hello Mr.Lawarchik,
Thank you very much for your very elaborative explanation.
Regards
We have unicoat coil coating system.It is Polyester amino system.There is no primer.Substrate is GI steel pretreated .
DFT of paint is 12-14 microns.How much QUVa hours it should remain fairly good
Good day.
First please define by what you mean by “how much QUV A hours it should remain fairly good”.
There are several criteria by which the durability of a coating accelerated weathering is determined, including gloss retention, color change, adhesion, blistering and film integrity. Accordingly you will need to define your criteria. Secondly, there are multiple types of resin systems comprising the description “polyester-amino plast coil coating” that can vary greatly in their weathering characteristics. Thirdly, the color of the coating also makes a large difference as for example pastel colors and whites normally provide better accelerated weathering properties than do dark colors. Fourthly the quality of the pigments used to obtain a color match can also contribute to performance. Fifth, there are multiple QUV A accelerated weathering tests as well as accelerated weathering conditions. For example ASTM D4587 Fluorescent UV-Condensation Test (AKA QUV) has 4 different cycles that provide somewhat different results depending on the cycle selected.
Lastly, I would not recommend especially a 12 – 14 micron thick single coat polyester over pretreated galvanized steel as such a thin single coat film will not provide a good barrier to moisture. The thin single coat film may result in blistering and adhesion loss depending on the exposure environment in a relatively short period of time.
Hi Sir, I need your time technical expertise in solving failure we observe in QUV Test after 500 hours. We are manufacturer of decals wherein we print inks and pu overcoat on pvc film. At 500 hours some spots are observed, however no discoloration is observed. Need your support.
Dear Mr. Kahn,
The spots you observe could be caused by a number of issues. Please contact me directly at [email protected]. 810-599-7286. We have a cadre of analytical and test equipment which we can utilize to solve the problem you see.
Thank you,
Ron Lewarchik