
Ambient curing by definition relies on conditions that are available in an ambient environment such as moderate temperature, natural light, moisture and air. From the use of ochre based cave paints 40,000 years ago to that used by the early Egyptians about 4,000 years ago that comprised pigment, wax and eggs; humans have been searching for and developing new chemistries and ingredients to provide improved performance of coatings applied at ambient conditions.
Comprised of natural occurring pigments and drying oils from linseed, poppy seed, walnuts and safflowers, the first ambient cure crosslinked paints were first used by Indian and Chinese painters between the fifth and 10th centuries.
The proper use of curing agents (either or both single or two component types) can provide improved:
- Chemical resistance
- Moisture resistance
- Adhesion
- Hardness
- Corrosion resistance
- Weather resistance
This article will cover only the general considerations of ambient curing agents with emphasis on newer chemistries or chemistries less often utilized. As there are previous Prospector articles concerning conventional epoxy two component (2K) coatings, two component coatings polyol-isocyanate technology and finally moisture cure silane functional crosslinkers and coupling agents, these technologies will not be discussed herein.
Looking for crosslinkers?
Prospector® allows you to search technical data on hundreds of materials. Find just what you need quickly and easily.
Create your free account now!
Isocyanate-free polyurethane chemistry
According to the California Department of Public Health, exposure to isocyanates can cause asthma. Occupational asthma has overtaken asbestosis as the leading cause of new work-related lung disease. In the last few years isofree technologies have emerged that do not utilize isocyanate crosslinkers to form a polyurethane and thus eliminate isocyanate exposure. Isofree 2K technology utilizing polycarbonate and polyaldehyde for example includes improved sprayable pot life, rapid cure and early hardness. Technologies that form polyurethanes without the use of an isocyanate crosslinker follow:
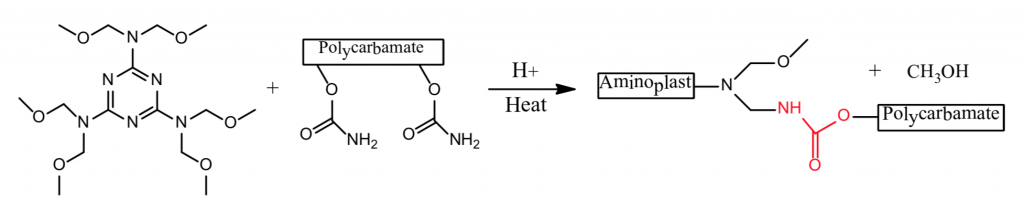
1. Hexamethoxy methyl melamine + Polycarbonate ⇒ Polyurethane
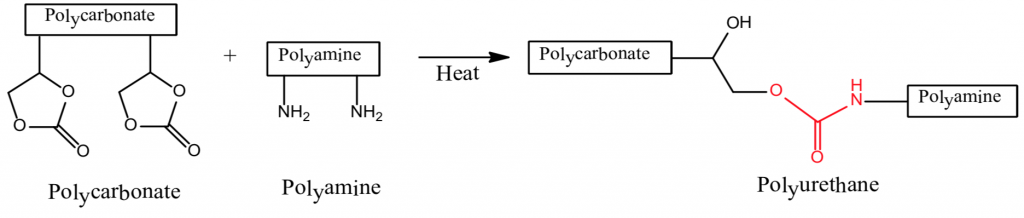
2. Polycarbonate + Polyamine ⇒ Polyurethane
3. Polycarbamate + Polyaldehyde ⇒ Polyurethane
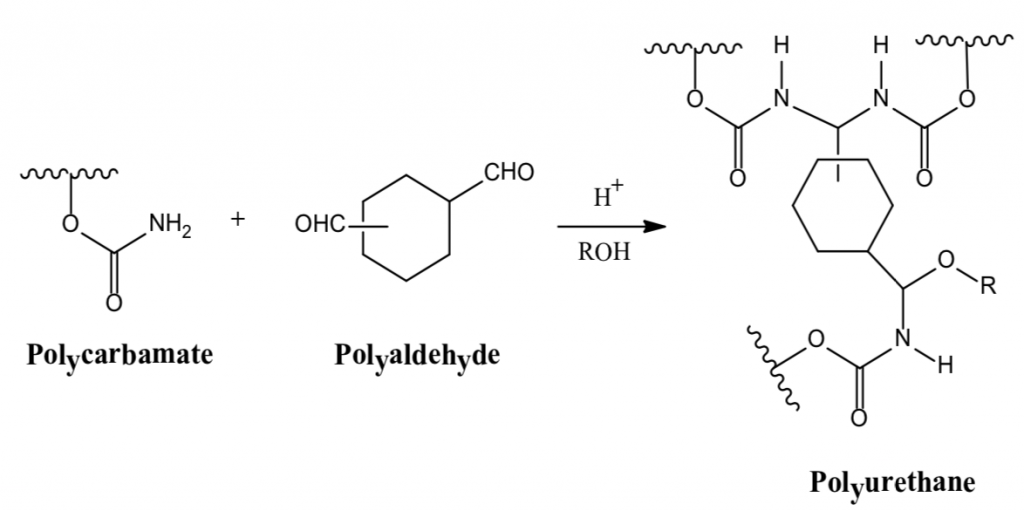
The formation of polyurethanes in reactions 1 and 2 are sluggish at room temperature, whereas the reaction rate of #3 that utilizes the crosslinking reaction of a polycarbonate and a polyaldehyde is more facile. Polyurethane formation by this reaction route provides a longer sprayable pot life and at the same time a faster reaction rate after application than that provided by the use of an isocyanate crosslinker.
Ketimine-Epoxy
One approach to provide stable epoxy-amine single component coatings is to utilize a blocked amine crosslinker. Primary amines react with ketones to form ketimines. Ketimines do not readily react with epoxy groups. In the presence of water, ketimines release the free amine plus ketone which is the reverse reaction of ketimine formation. Normally methyl ethyl ketone is used which upon application volatilizes quickly under ambient conditions, the amine then reacts with the epoxy to form a cured film. A moisture scavenger additive can eliminate the reaction with water prior to application.

Ketimine-epoxy systems are indefinitely stable in the absence of water and can thus permit one component systems.
Crosslinking with unsaturated groups
- Acrylated oligomers can be used as a crosslinker to crosslink polyfunctional amines through a Michael Addition Reaction. As this reaction is fast, blocked amines can be used (ketimines). Once the ketimine is unblocked in the presence of moisture, it forms a primary amine that adds to the acrylate for the reaction of a primary amine and an acrylate. See illustration below.
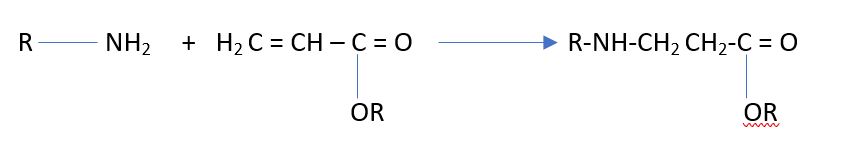
- Acrylated oligomers can also be crosslinked using the Michael Addition Reaction with acetoacetoacetylated resins and their enamine analogues.
- Vinyl polymerization – Coatings utilizing acrylated and/or methacrylate oligomers and suitable unsaturated polyesters (using fumerate and/or maleate groups) can be utilized in two component systems with the addition of a suitable free radical initiator such as methyl ethyl ketone peroxide and accelerators such as cobalt napthenate and dimethylaniline.
Other common crosslinking reactions utilized in ambient cure coatings
| Hardener
Cross-linker Functional Group |
Resin
Cross-linkable Group |
Cross-linked group |
| Polyaziridine
|
R-COOH
(carboxyl) |
Acetyl urea |
| Silane
Triethoxy silane and aliphatic epoxy
|
Dual self-cure mechanism | Siloxane & epoxy ester |
| Carbodiimide
R-N=C=N-R |
R-COOH | N-Acyl Urea |
| Isocyanate prepolymer
R-NCO |
R-OH
(hydroxyl)
R-NH2 (amino generated from reaction of water with isocyanate) |
Urethane
Urea |
| Hydrazide
|
R-C=O
Ketone |
Hydrazone |
Sources:
- Prospector Knowledge Center: Chemistry of resins and hardeners
- Prospector Knowledge Center: Superior performance with organosilane chemistry
- Prospector Knowledge Center: Patently innovative latest polyurethane technologies
- Prospector Knowledge Center: Polyisocyanates deep dive
- Prospector Knowledge Center: Reactive silanes for enhancement of coating performance
- Prospector Knowledge Center: Ambient cured latex paints and how to improve performance
- California Department of Public Health: Isocyanates: Working Safely [PDF]
- Wikipedia: Michael reaction
- Web exhibits.org
- Dow Isocyanate Free Polyurethane Coatings, European Coatings Conference, April 21, 2015
- Organic Coatings, Science and Technology, Third Edition, Wiley, Wicks e.al. 2007
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.




Dear Ron,
and with respect to powder coating, what it’s the best curing or hardener for polyester and polysiloxanos?
I hope your comments with very much interesting.
br
carlos