 Many reactions in the resins and coatings industry require a catalyst. But what is a catalyst?
Many reactions in the resins and coatings industry require a catalyst. But what is a catalyst?
To fully understand this, a brief introduction to thermodynamics is helpful. Reactants in a two-component system, for instance, are at a certain energy level. If the resulting products are at a lower energy level, the reaction is called exothermic. Nice examples are isocyanate/hydroxyl and acid/epoxy. If the products are at a higher energy level than the surfactants the reaction is called endothermic. Normally they are not very helpful in the industry.
In all cases, a so-called activation energy is required, since both reactants and products are stable. During the process, however, a so-called instable intermediate is formed. The energy path has to pass a “hump”. This is where a catalyst steps in. It lowers this “hump”:
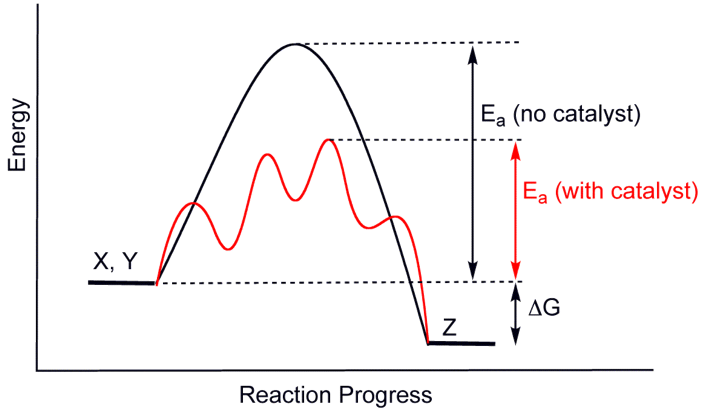 Two phenomena are clearly visible.
Two phenomena are clearly visible.
First of all, the reaction pathway when using a catalyst has more than one maximum. This is due to the fact that the catalyst lowers the energy level of the intermediate by dividing the process over several steps:
- Attaching to the reactants forming a complex often with a specific charge distribution,
- Performing the actual reaction, and
- Dissociation of the catalyst from the product(s)
Secondly, it is clear that a catalyst not only lowers the activation energy going from reactants to products, but also lowers the activation energy going from products to reactants again, e.g. “the wrong way.”
As an example, a catalyst is used in the production of polycondensates, a process yielding polyesters and water. If this polycondensate is then later emulsified in water, van‘t Hoff’s law of equilibrium1 predicts that the polycondensation will be reversed via hydrolysis. This occurs especially due to the huge amount of water molecules: water has a low molecular weight – 18 – so a small amount can represent a huge number of molecules. Thus, when using a catalyst for the preparation of a polyester or alkyd to be emulsified, be aware that it will be more susceptible to hydrolysis.
Catalysts are usually strong amines like tetramethyl Guanidine (TMG, for transesterification), although also weak amines (triethylamine for urethane crosslinking) have found their use in the coatings industry.
On the other hand, strong acids are also used for different crosslinking mechanisms. For instance, the process of etherification (two alcohol moieties reacting, yielding an ether and water) is very efficiently catalyzed by strong acids like p-toluene sulphonic acid (p-TSA).
When a catalyst is neither a base nor an acid, it usually is a heavy metal and as such sometimes under scrutiny. Examples are:
- Tin, used in urethane based two-components systems, tends to be more and more replaced by less harmful zinc or bismuth.
- Antimony oxide, which is used for transesterification (ester bonds being replaced by other ester bonds), to yield PET. A very useful replacement in this case is the strong base TMG mentioned before.
Once the catalyst has done its job, it leaves the products in search for new reactants to lower their activation energy. It has not changed. This is the most important feature of a catalyst: it is not consumed in the reaction. This means that only small amounts are necessary, which is a good thing since they are not cheap. Prices far above $4/pound are no exception. Starting quantities are therefore usually around 0.1%, of course to be optimized.
Finally, the following table provides an overview of resin/coating chemistry and their catalysis:
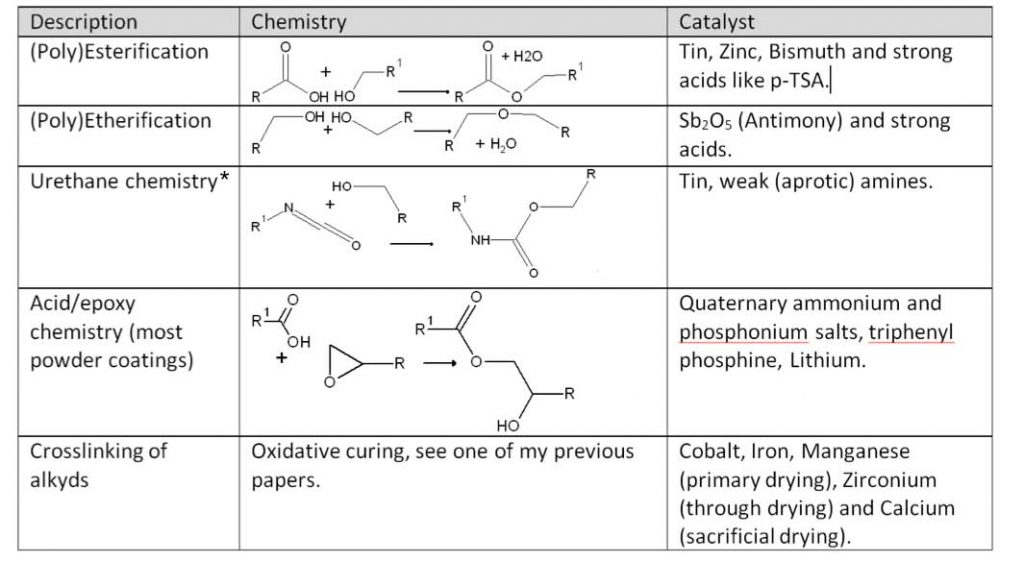
*The Urethane chemistry example shows only the reaction with an alcohol. Isocyanates also react strongly with amines and carboxylic acids at body temperature, and hence are very toxic.
Reference:
- Le Chatelier’s Principle and the laws of van’t Hoff [PDF]
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Hallo … great article! … especially vant Hoff …
– do you know typical Provider of These catalysts? …
– hydrogenation process is not important for resins?
… thank you for a short comment
Julius Nickl
http://www.candcs.de
Dear Ad,
very clear and good to read! BR. Daniela
Good day, I would like to know how can I contact the author for a business proposition.
Best regards
Good summary!! Thanks.
Do you have any document about crosslinking of alkyds (oxidative curing)
Can I have It?
Contact me through LinkedIn.
Kind regards,
Ad
Do you have any document about crosslinking of alkyds (oxidative curing)
Can I have It?
Very good for Knowledge Enhancement,Thanks a lot.
Dear sir,
I wan to read your other articles on polyester resin for powder coating.we are manufacturing powder coating powder in India under brand Purecoat .
We appreciate if you please give list of other publications.