 edited Nov. 12, 2021
edited Nov. 12, 2021
Original article date: Oct. 7, 2016
Introduction
Several problems can occur with liquid systems that contain solid particles like pigments and fillers. One of the undesired phenomena is flocculation, the gluing together of solid particles in a liquid1.
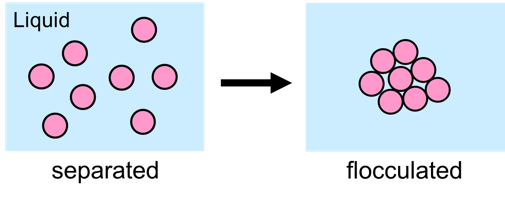
Flocculation is caused by the attractive forces that are always present between particles. The principle used to prevent flocculation is to assure that the particles repel each other. The repulsive forces must be stronger than the attractive forces. Next to steric stabilization2, electrostatic stabilization can be used as a mechanism to arrange repulsion.
Electrostatic stabilization
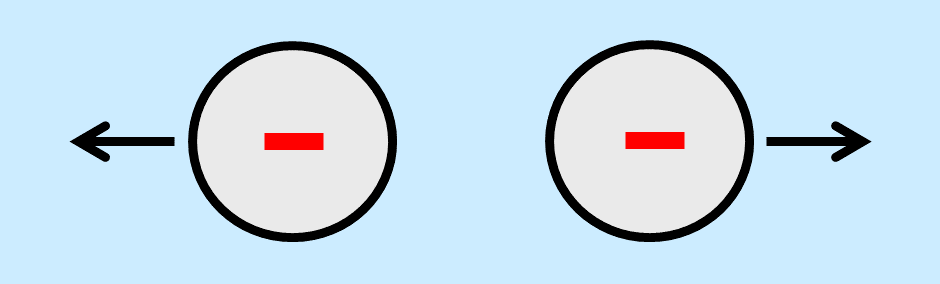
Flocculation is prevented by means of electrostatic stabilization when all particles in a system carry an electrostatic charge of the same sign. The amount of charge on the particles must be sufficient to overrule the attractive forces. In most paints and inks, the particles have a negative charge. This type of stabilization, called anionic stabilization, is most often used in water-based systems.
Polymeric dispersants
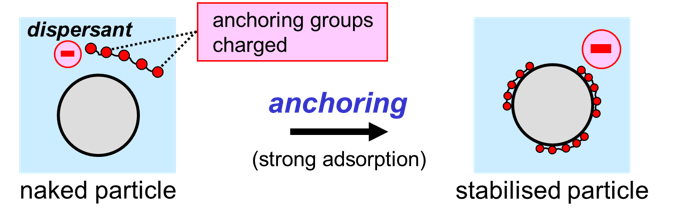
The solid particles themselves can be charged, but most often, product developers assure that all particles have an identical electrostatic charge by using charged polymers that adsorb at the surface of the particles. A polymeric additive that provides stabilization against flocculation is called a dispersant3,4. Dispersant molecules must have two sorts of groups to be able to provide electrostatic stabilization. First, anchoring groups must assure that the molecules adsorb at the surface of the particles. Secondly, the dispersant molecules must contain groups that have a negative charge. Solid particles that are surrounded by water will obtain a negative charge when a dispersant is used that complies with these criteria.
An example
Lopon® 890 is an anionic dispersant used for electrostatic stabilization of solid particles in water-based systems. The additive consists of linear polyacrylate molecules dissolved in water. The molecules carry a negative charge because part of the acid groups (-COOH) of the polymer are neutralized with sodium hydroxide (NaOH).

The acid groups and the carboxylate groups (-COO–) act as anchoring groups, and the carboxylate groups provide the negative charge.
Critical aspects of electrostatic stabilization
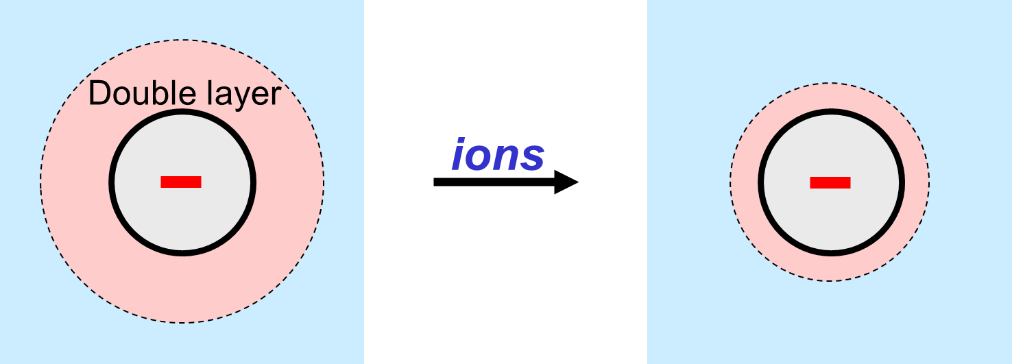
The formulator must be alert when using electrostatic repulsion as the only stabilization mechanism. The reason is that stabilization via charge is sensitive towards possible changes in pH: the charge of (the dispersant molecules on) the particles depends upon pH. Also, the repulsion of charged particles depends upon the presence of salts. The mutual repulsion of charged particles becomes worse when ions are present in the water surrounding the particles. This is because the charged layer around the particles, the so-called electric double layer, shrinks when ions are added to the water. The double-layer must have a certain minimum thickness to assure sufficient repulsion.
References
- Article The Basics of Dispersion and Stabilization of Pigments and Fillers, Jochum Beetsma, 31 January 2020.
- Article Dispersants for Steric Stabilization, Jochum Beetsma, 1 October 2021.
- Article Dispersants – The Understood Misunderstood Additive, Marc Hirsch, 10 September 2021.
- Article Understanding Dispersants, Marc Hirsch, 19 February 2016.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Dear Jochum Beetsma,
We are impressed with your expertise in the coating and ink Industries.We are looking for Mutifunctional dispersing agent incorporating negative charge to viscose during its manufacturing stage.The product should be stable to alkali and and acid during regeneration of viscose fibre.Your suggession will be highly appreciated
Dear Karur,
Thanks for your kind response. The information in your explanation is too limited to give an advice. Could you please send me a mail ([email protected]) so that we can discuss the topic ‘privately’?
Thanks for your mail on forehand,
Jochum Beetsma.
like to get small sample.
Hi Leo,
I am the Knowledge Center content manager. You can request samples of many specific materials via the Prospector search engine.
Thanks!
We do have very good experience with carboxylated resins, because you not only get excellent steric stabilisation but in addition high glossy coatings.
Thanks you so much for the information. Please clarify to me the criteria for selecting either an electrostatic or steric hindrance stabilising dispersant. What would be the advantage of choosing one vs. the other?
Dear Annie, most important is the type of stabilisation used for the binder dispersion in the system: it is important that all particles/droplets in a paint are stabilised in the same way.
Advantages of steric above electrostatic:
– Less sensitive towards ions (salts) in the water and towards changes in pH.
– Better in freeze-thaw stability.
– Applicable in both waterbased and solventbased systems.
Disadvantage of steric compared to electrostatic:
– More dispersant needed.