What do deep frying and painting with alkyd resins have in common?
They both use polyunsaturated fatty acids (PUFAs), like soybean oil. These PUFAs can be oxidized by airborne oxygen (constituting approximately 20 percent of the air surrounding us) to polyunsaturated hydroperoxides.
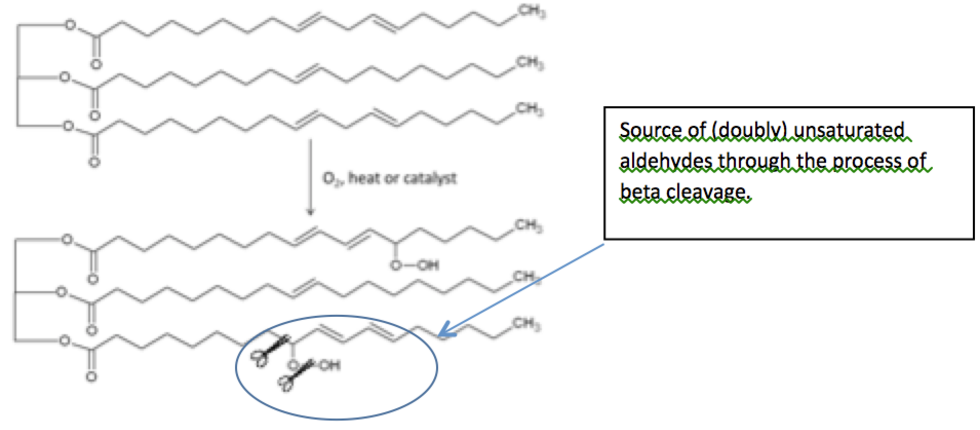
This process is catalyzed in alkyd paints by multivalent metal soaps, but it occurs spontaneously when deep frying at 220°C under atmospheric conditions.
However helpful these hydroperoxides are in the drying of alkyd paints, they can also decompose to harmful saturated and doubly unsaturated aldehydes, some of which may even be carcinogenic, as has been shown by Grootveld et al.
The situation for deep frying is even worse. Suspicion of certain forms of carcinogenic activity has been reported after investigating workers in fish-and-chip shops, where dealing with a deep-frying pan is a daily job. The main suspect is an oxidation product of linoleic acid: α, ß, γ, δ -Decadienal.
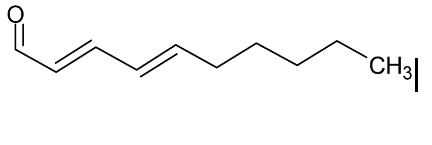 This very reactive compound will immediately be released at 220°C from a deep-frying pan.
This very reactive compound will immediately be released at 220°C from a deep-frying pan.
The good news is that hardly any of the nasty material will remain in the food.
Even better news is that this polyunsaturated aldehyde is so reactive that it is very unlikely it will escape an evenly reactive matrix like a drying alkyd. It most certainly will co-react with radicals playing an intermediate role in a drying alkyd paint.
Fortunately, the same goes not only for α, ß, γ, δ –Decadienal, but also for other unsaturated aldehydes.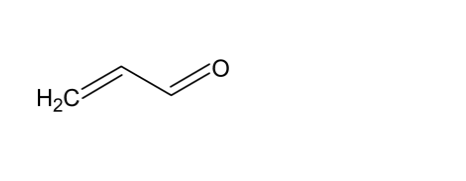 In drying alkyds based on linolenic acid, another reactive compound, acrolein (above), can been seen due to its high vapour pressure, but only at very low ppb levels. Happily, linolenic acid is very seldom used in alkyds due to its tendency to cause yellowing.
In drying alkyds based on linolenic acid, another reactive compound, acrolein (above), can been seen due to its high vapour pressure, but only at very low ppb levels. Happily, linolenic acid is very seldom used in alkyds due to its tendency to cause yellowing.
It is good to know that aldehydes and their emissions are caused by the start of the oxidative drying process, where hydroperoxides are formed. Let’s call that process initiation drying.
Other radical polymerization processes within an alkyd-like system where oxygen is not involved, we’ll designate propagation drying. That means aldehydes are not formed. Moieties that participate in this type of non-oxidative polymerization are methacrylic, fumaric and itaconic and also conjugated fatty acids. (Muizebelt has done quite a lot of work on this.3)
During propagation drying, not only are there no aldehydes formed on the parts that break off, but also no aldehydes form on the trunks. Since these α, ß, γ, δ –aldehyde trunks are the main cause of yellowing, it can be expected that the use of conjugated fatty acids also reduces an alkyd’s tendency for yellowing.
But that’s enough on the drying of alkyds. Let’s stick to deep frying.
The main aldehydes produced by heated cooking oil are:
- Omega-3 fatty acid from linolenic acid (linseed oil) will yield propanal (propionaldehyde) and many unsaturated derivatives because it has three double bonds, of which two are conjugated, left after the hydroperoxide formation.
- Omega-6 fatty acid from linoleic acid (soybean and sunflower oil) will yield hexanal (hexanaldehyde) and few unsaturated derivatives because it has only two conjugated double bonds left after the hydroperoxide formation.
- Omega-9 fatty acid from oleic acid will yield nonanal (nonanaldehyde) but no unsaturated derivatives.
If only everything in life could be so simple.
In summary, deep fry in saturated fats and oils as much as possible. Olive oil and lard are good examples. Of course, when there is no heating involved, sunflower oil is the best choice.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Nice overview of an established chemistry that still finds many novel tweaks for new applications. Readers may be interested in United Soybean Board: http://unitedsoybean.org/