Emulsion polymerization was developed by The Goodyear Tire & Rubber Company in the 1920s. The emulsion-polymerization process results in a latex particle, which is a dispersion of polymer in water. Waterborne coatings that primarily use emulsion polymers are the largest type of coating technology used on a global basis and are expected to continue to grow as a percent of the total coatings market.
In emulsion polymerization, monomers are first dispersed in the aqueous phase. Initiator radicals are generated in the aqueous phase and migrate into the soap micelles that are swollen with monomer molecules. As the polymerization proceeds, more monomers migrate into the micelle to enable the polymerization to continue.
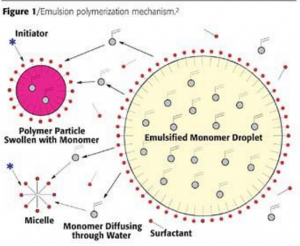
Since only one free radical is present in the micelle prior to termination, very high molecular weights are possible., on the order of 1,000,000 or higher. Unlike solution polymers, the viscosity of latexes are governed by the viscosity of the medium the particles are dispersed in (continuous medium). Chain transfer agents are added to control the molecular weight. The resultant emulsion particle is an oil in water emulsion. monomer in the aqueous phase.
A less commonly used emulsion technique called the inverse emulsion-polymerization process involves dispersing an aqueous solution of monomer in the nonaqueous phase.
Emulsion polymerization can occur using a batch process, semi-continuous process or continuous process. Commercial latex polymers are made using a semi-continuous or continuous process rather than a simple batch process because the heat evolved in a simple emulsion batch process would be uncontrollable in a large reaction vessel. In the semi-continuous batch process, monomers and initiators are added in proportions and at a controlled rate so that rapid polymerization occurs. In this method, the monomer concentration is low, also called under-starved monomer conditions, to facilitate temperature control. It is also common to start the polymerization using a seed latex.
In the continuous process, the reaction system is continuously fed to, and removed from, a suitable reactor at rates such that the total volume of the system undergoing reaction at any instant is constant.
Mini-emulsions are produced by dispersing monomers in water by means of vigorous mechanical agitation or homogenization using a mixed emulsifier system,  including a classical emulsifier and a water-insoluble co-surfactant, such as a long-chain fatty alcohol or alkane (e.g. cetyl alcohol or hexadecane). The final polymer particles have almost the same size as the initial monomer droplets. Their particle size distribution is broader than those obtained by conventional means.4.
including a classical emulsifier and a water-insoluble co-surfactant, such as a long-chain fatty alcohol or alkane (e.g. cetyl alcohol or hexadecane). The final polymer particles have almost the same size as the initial monomer droplets. Their particle size distribution is broader than those obtained by conventional means.4.
Table I. Raw Material Selection for Emulsion Polymerization
| Material | Example(s) | Considerations and Comments |
| Monomers | – Methyl methacrylate
– Styrene – Vinyl esters – Methacrylic acid |
– Monomers that do not react with water
– Monomers that undergo free-radical chain polymerization – Monomers with limited water solubility – Carboxyl functional monomers used in low levels for particle stability – Hydroxyl functional monomers for reactivity with crosslinker – Monomer compatibility and rate of polymerization |
| Initiators | – Sodium, ammonium or potassium persulfate
– Azobisisobutryl nitrile (AIBN) – t-butyl hydroperoxide (TBHP) |
– Must be water soluble for REDOX type initiators (persulfates)
– Thermal initiation for free-radical types (e.g. AIBN, TBHP) |
| Surfactant | – Anionic and nonionic types
– Anionic types – Sodium lauryl sulfonate – Nonionic types |
– Limited water solubility
– Toxicity issues with nonylphenol ethoxylates |
| Other ingredients | – Water
– Buffer (e.g. sodium bicarbonate) – Amine – Coalescent |
– Normally deionized water to minimize variability
– Buffer to regulate pH – pH control, stability – Aids film formation |
In micro-emulsion polymerization, the initial system consists of monomer droplets varying from 10 to 100 nm dispersed in water with the aid of a classical emulsifier, such as sodium dodecyl sulfate (SLS), and a co-surfactant, such as a low molar mass alcohol (pentanol or hexanol). Micro-emulsions are thermodynamically stable and optically a one-phase solution. Reaction rates are normally very fast. The micro-emulsion polymerization process produces latex particles that are less than 50 nm. The average micro-emulsion particle contains only one polymer molecule with average molecular weight exceeding one million.3
Table II – Characteristics of Various Waterborne Resins 1
| Resin Type | Major Characteristic(s) |
| Latex | Particle size of 5 nm – 10 microns, opaque liquid |
| Water Reducible | Reduction with water results in irregular viscosity vs. solids relationship |
| Polyurethane Dispersion (PUD) | – Particle size of 1-20 microns – Lower Minimum Film Forming temperature in relation to dry film Tg
– Lower cosolvent demand |
| Miniemulsion | – 50-500 nm particle size |
| Microemulsion | – Particle size of 10-100 nm,
clear liquid |
| Core-Shell (Low Tg shell with high Tg inner core) | – Reduced blocking
– Lower coalescent demand |
Additional information on waterborne coatings can be found in the following Prospector articles:
- Fundamentals of Waterborne Resin Technology
- Improving Performance in Ambient Cured Latex Paints
- Dispersing and Wetting Hydrophobic Pigments and Fillers in Water-based Paints to Avoid Pigment Floating and Flooding
- Lewarchik, R. Fundamentals of Waterborne Resin Technology. 2015 Sept 18. Overland Park (KS): UL Prospector; [accessed 2016 Jun 8]. https://www.ulprospector.com/knowledge/3069/pc-fundamentals-waterborne-resin-technology/
- Nanostructured, Nonuniform and Core-Shell Polyurethane Dispersions. 2005 Oct 1. Troy (MI): PCI Magazine; [accessed 2016 Jun 8]. http://www.pcimag.com/articles/83072-nanostructured-nonuniform-and-core-shell-polyurethane-dispersions
- Micro-Emulsion. c2016. Derby (UK): Enviroquest; [accessed 2016 Jun 8]. http://www.enviroquestgpt.co.uk/content/micro-emulsion.html
- Poehlein, GW. Encyclopedia of Polymer Science and Engineering, 2nd Edn., Mark, HF, et al., Eds. 1986. Vol. 6: 1
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Very interesting article.
I’m just missing one monomer which is as well used on low levels as a stabiliser of the emulsions.
I’m talking about a stronger acid than carboxylic. It is vinyl sulfonic acid, applied as sodium salt.
Best regards
Claus Neufeld
BU Mgr Functional Polymers
Proviron
Nice job, Ron. A lot of information to convey in a short article. The only thing I could think to add is carboxyl-functional monomer usage above the 1-3% used for emulsion stabilization. Usage at higher levels to impart the functionality for cross-linking.
Brief but perfect! I found this article very useful in the principles of emulsion polymerization
“Usage at higher levels to impart the functionality for cross-linking.”
Does that mean higher level acid is harmful to crosslinking process?
Concise informative article Ron. Thanks!!
Steve Johnson
Business Development Manager
Halltech Inc.