 Effect pigments provide an infinite array of colors and effects that enable unlimited design possibilities for coatings. These effects include the illusion of flickering lights, metallic reflection, interference sparkle and color variation and luster that changes with the viewing angle and light source.
Effect pigments provide an infinite array of colors and effects that enable unlimited design possibilities for coatings. These effects include the illusion of flickering lights, metallic reflection, interference sparkle and color variation and luster that changes with the viewing angle and light source.
They are used in a variety of coatings, including those in automotive, monumental and smaller buildings as well as other industrial and product finishing applications. Pigments may be broadly classified by their ability to reflect light: absorption, metallic and interference.
Conventional organic and inorganic pigments are classified as absorption pigments, because they absorb certain wavelengths of the incident light that strikes their surface. The sensation of color is produced by the remaining component of the reflected visible light that produces the color we observe.
For example, a quinacridone red pigment reflects the portion of the light that produces a red color and absorbs the rest of the light energy. Titanium dioxide reflects all of the light and absorbs none, while carbon black absorbs all and reflects none. Due to their ability to absorb light, absorption pigments do not display a metallic luster or iridescence and are thus one dimensional in their ability to interact with light.
Metallic pigments consist of tiny flat pieces of aluminum, bronze, zinc, copper, silver or other metals that reflect light and thus create a metallic luster. These pigments are two- dimensional or metallic pigments.
Looking for effect pigments for your coatings formulation?
Prospector has listings for effect pigments from global suppliers. View technical data, request samples and more now.
Get Material Data
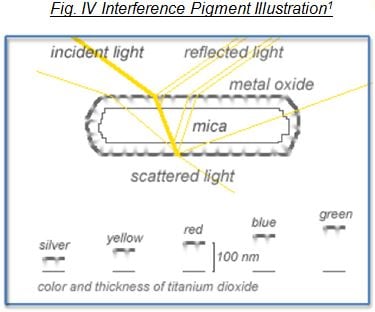
Interference pigments consist of various layers of, for example, a metal oxide deposited onto mica, a natural mineral. Light striking the surface of these pigments is refracted, reflected and scattered by the layers that make up the pigment. Through a superimposition (or interference) of the reflected rays of light, a changing array of color is created, with the most intense color seen at the angle of reflection.
Effect pigments are unique in respect to how they interact with light due to their geometry which is normally a platelet with a high aspect ratio (ratio of width to height). Depending upon the specific technology, a wide variety of colors and effects can be created, such as interference shimmers, color travel effects or metallic reflection.
Metallic Flake Pigments are platelet in structure with a chemical treatment for solvent born paints and a different treatment to stabilize the metal for waterborne coatings. Metallic flakes can be copper and zinc, but most often aluminum due to it’s high reflectivity.
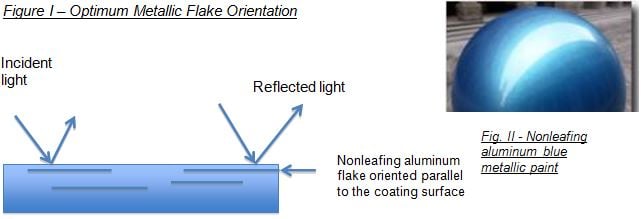
In a properly applied coating, these mirror-like pigments orient parallel to the surface and thus offer optimum reflectance (figure I). Leafing aluminum on the other hand is surface modified to “float” the aluminum to the the coating surface and provide the appearance of solid aluminum.
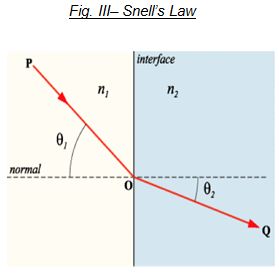 Snell’s Law states that when incidence light travels through one medium into another, the refracted light may change it’s angle of travel. The refracted angle relative to the incident angle in air is called the Index of refraction. It is these principals that are operative to produce interference pigments that produce a myriad number of colors and effects.
Snell’s Law states that when incidence light travels through one medium into another, the refracted light may change it’s angle of travel. The refracted angle relative to the incident angle in air is called the Index of refraction. It is these principals that are operative to produce interference pigments that produce a myriad number of colors and effects.
Depending on the platelet or laminar substrate that is employed to deposit another layer onto the substrate as well as the material choice and thickness of the deposited film, a wide variety of special effects can be produced.
 Pearl Pigments can be produced by deposition onto two major classes of substrates, natural and synthetic. Natural substrates include mica and Kaolin. Synthetic substrates include alumina, borosilicate, silica and synthetic mica. The coating on alumina and silicates is anatase titanium dioxide that may or may not be doped with another metal to create an even greater number of additional colors and special effects.
Pearl Pigments can be produced by deposition onto two major classes of substrates, natural and synthetic. Natural substrates include mica and Kaolin. Synthetic substrates include alumina, borosilicate, silica and synthetic mica. The coating on alumina and silicates is anatase titanium dioxide that may or may not be doped with another metal to create an even greater number of additional colors and special effects.
Other special effect pigments include luminescent pigments that emit light (visible, IR or UV) upon suitable excitation, without becoming incandescent. Phosphorescent pigments emit light by a luminescent pigment after excitation has ceased (e.g., glow-in-the-dark). Thermochromic pigments reversibly alter color after exposure to a heat source, and lastly photochromic pigments reversibly alter color upon exposure to a strong uv light source. Luminescent, phosphorescent, thermochromic and photochromic colorants and pigments are not widely used in decorative coatings applications. They are only used for specialty purpose applications, as they are classified as special effect pigments.

 For additional information on other types of pigments, please see “Understanding Corrosion Inhibitive Pigments” as well as “Inert Pigments – The Unseen Contributor to Improving Paint Performance.”
For additional information on other types of pigments, please see “Understanding Corrosion Inhibitive Pigments” as well as “Inert Pigments – The Unseen Contributor to Improving Paint Performance.”
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Nice article, Ron. I’ll tie this in to an upcoming one I am writing on Functional pigments so this is a great lead-in.