Although alkyds are no longer the largest volume resin type used in coatings, they still play a significant role in the coatings industry, not only because of their versatility, but also because they employ a significant amount of renewable material. The term alkyd is derived from alcohol and acid. Alkyds are prepared from the condensation reaction between polyols, dibasic acids and fatty acids. The fatty acid portion is derived from vegetable matter and thus is a renewable resource. Key performance features of alkyds include their ability to offer improved surface wetting (from the bio-based fatty acid portion of substrates and pigments) and lower cost (also primarily from the fatty acid portion). The most widely used polyols include glycerol, pentaerythritol and trimethyol propane whereas the most widely used dibasic acids are phthalic anhydride and isophthalic acid.
Looking for Alkyd Resin for your fomulation?
Prospector has thousands of plastics materials from global suppliers. Find technical data and request samples now!
Search Alkyd Resins
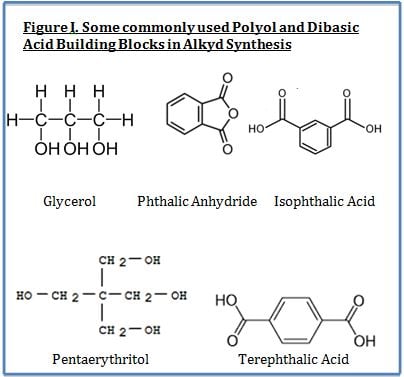
Naturally-occurring oils are in the form of triglcerides. Triglycerides are triesters of glycerol and fatty acids. Triglycerides can be drying oils, but many are not. The reactivity of drying oils with oxygen results in 1,4 –dienes. The naturally-occurring oils are comprised of mixtures of mixed triglycerides with different fatty acids as part of the glyceride molecules.
Some of these glyceride molecules are comprised of a higher percentage of fatty acids with a greater amount of non-conjugated unsaturation with diallylic methylene groups and result in improved drying capability. For example, linoleic acid has one active diallylic group (-CH=CH – CH2 – CH=CH -), whereas linolenic has two active methylene groups. Also, to increase drying speed, alkyds can be modified with vinyl toluene or styrene to increase the Tg and thus reduce the time required to reach a given hardness. If the amount of oil in an alkyd is over 60%, it is called a long oil alkyd. If it’s between 40 and 60%, it’s known as a medium oil alkyd, and those with less than 40 are considered short oil alkyds. The formula for calculating the percent oil length based on the amount of fatty acid is as follows:

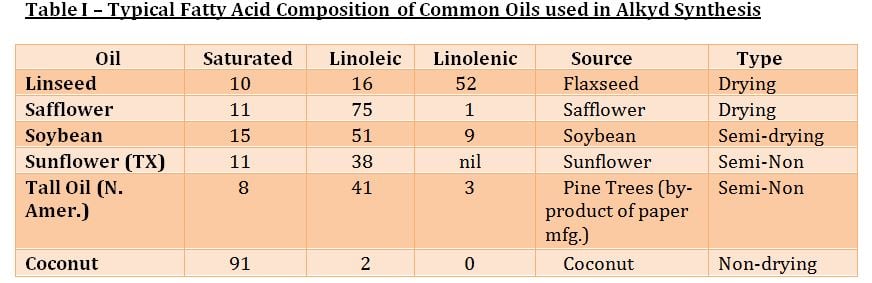
In addition to the amount of oil as well as the selection of the alcohol and acid functional components, the type of oil has a profound effect on the dry time and performance.
Fatty acids are further categorized into drying, semidrying and non-drying. Non-conjugated oils are considered drying oils if their drying index, as calculated as follows, is more than 70. The higher the amount of Linolenic and Linoleic content, the higher the drying index:
Although drying speed is improved as the % linolenic increases, the rate of yellowing for exterior white coatings is also greater. Accordingly, alkyds using safflower and sunflower oils which provide improved resistance to yellowing as a result of their lower linolenic content.

In addition to classifying alkyds by their oil length and the type of fatty acid present, alkyds are also classified into oxidizing and non-oxidizing categories. Oxidizing alkyds crosslink through a complex multistage auto-oxidation mechanism, whereas Non-oxidizing alkyds do not crosslink and are thus thermoplastic unless their available hydroxyl groups are crosslinked with an aminoplast (heat cured) or isocyanate crosslinker (ambient cured).
Alkyd resins are produced by three main processes¹:
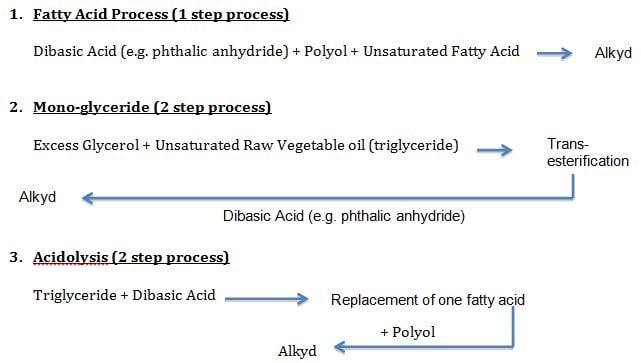
In the fatty acid process where the composition of the resulting resin can be more precisely controlled, an acid anhydride, a polyol and an unsaturated fatty acid are combined and cooked together until the product has achieved a predetermined level of viscosity. The monoglyceride process is a two step process where an excess of glycerol is used in conjunction with an unsaturated raw vegetable oil to first transesterify the oil and then a dibasic acid is added in the second step to form an alkyd. In the acidolysis process a triglyceride is first reacted with a dibasic acid to replace one of the fatty acid groups, and then in the second step a polyol is added to form the alkyd resin.
In a formulated paint, autooxidation of oxidizing alkyds is slow unless catalyzed, accordingly metal salts are added to accelerate cure. Historically the most widely used driers are oil soluble salts of 2-ethylhexanoic acid or naphthenic acid with lead, cobalt, manganese, zirconium and calcium. Cobalt and manganese salts promote surface dry, whereas lead and zirconium promote through dry. Lead and cobalt based driers have toxicity issues and have been largely replaced by less toxic driers. Calcium salts do not show much catalytic activity, but are used to reduce the amount of surface and through driers as well as to assist pigment wetting. In general, a ladder study should be undertaken to optimize the level of driers, as, in addition to promoting cure, they also contribute to long-term embrittlement, discoloration and moisture sensitivity.
Alkyds are versatile and can be modified to fit multiple applications for use in coatings to provide low VOC or enhanced performance. For example, higher solids alkyds can be prepared by decreasing the dibasic acid to polyol ratio, using a higher percentage of oil or decreasing the molecular weight distribution. Water-reducible alkyds are available in the US and Europe that contain very little or no VOC. Some of these alkyds utilize surfactants to provide improved stability in water, while others are made by emulsifying the alkyd in hot water with the aid of an emulsifier. Waterborne alkyds can be more sensitive to hydrolysis of the ester linkage. Hydrolytically stability can be improved by employing an acrylic shell with an alkyd core. Lastly, uralkyds are also known as oil-modified urethanes as they are comprised of a urethane linkage and oil. These are made through transesterification of a polyol and an oil to form a monoglyceride and then reacted with a less than stoicheometric amount of diisocyanate. Uralkyds provide excellent mar, abrasion, chemical and saponification resistance and are used as maintenance paints and for wood application requiring these properties.
For additional information concerning bio-based resins and raw materials, please navigate to www.ulprospector.com.
[1] Encyclopedia of Polymeric Nanomaterials, University of Akron, Springer-Verlag, Berlin Heidelberg, 2014
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Mr.Ron Lewarchik,
Excellent Basics explanation was given.
N.SRIDHAR
Iam working in factory for alkyd resin and urethanted in Egypt, can l know what is the new in that field?
really resourceful!
whats the most recent and efficient alternative to alkyd?
In the past 12 months the alkyd we use here Nigeria has quadrupled in cost!
yes alternate available that can reduce your price upto 30% please contact with me on my email address [email protected]
I am working on Resin manufacturing unit, i want to know some information:
If acid value is not decreased during process and viscosity is finalised then what will be resin effect on paint. Also share some detail that how to drop acid value during process if its high from our standard.
Add glycerin in it 30 to 40 kg
Please contact
[email protected]
Add solvent for reflux and N2 gas up.
Hello,
Came across this article which is very nicely explained for core understanding by Ron.
I am getting issues of difference in color in batches while making a soya oil alkyd with the same formulation being used.
Can someone give a reason of this problem.
Thanks
Vishal
Hi Vishal,
Slight variations in time or temperature along with variations in the quality of your soya FA can create variations in color. A low level of triphenyl phosphite normally provides improved color in alkyds along with less variation in color. You can locate suitable suppliers on Prospector along with recommended levels once you contact the supplier.
Best regards,
Ron
Friends.
I was trying to make a low molecular weight alkyd and was successful with acid value less than 12 and also the oil length around 67% with FA and phthalic anhydride.
The viscosity and flow at 100% was comfortable and less than 120 KU.
However, when I draw a clear film of this on a glass plate, I find that there is a tendency of dewetting and full of pattern.
Any body has experienced this and also request your good self to give a solution to this. I need clean and defect free surface. (Application was done at 100 micron
Dear Sir , please explain how many types of alked resins
Plz update us about new technology used in alkyd resins
I want to manufacture alkyd resin by using terephthalic acid so what process should I prefer? What composition should I look for?
Please give me suggestions
How much percentege of benzoic acid use in formulation of Soya based Long oil Alkyd resin?
Benzoic acid is rarely used to make alkyds as it is a mono acid and as such does not build molecular weight. It is sometimes used in small proportions for specialized properties or to adjust functional group levels.
How to calculate R value and functionality of alkyd resin?
Can somebody tell me how to calculate %of soya fatty acids and pthalic anhydride where i from
Dear Sir,
I am working in a Resin Manufacturing Plant. Months before we have worked on decreasing batch cycle time and we did it successfully. Now BCT ranges between 16 to 18 hour, MG forms at 260C to 265C temp, We drop final resin at 230C.
In this condition we have been facing problem of too much deposit formation on the condeser. previously the deposit was made with Phthalic but now a days it forms with some finished product.
We are looking for a good solution for this problem.
Thanking in advance,
Md. Omar Faruk
The answer to your question requires additional dialogue and time commitment that would require a consulting agreement.
Interested in Use of short oil alkyds in preparation varnish.
I am happy to come in contact with this forum, I need a book that deal on Auto paint, and oil based formulation
HI SIR
WE PRODUCE LONG OIL ALKYD RESIN.
I HAVE QUSTION ABOUT ACID NO. IF ACID NO WAS 13 HOW DIFFICULT HAPPEN IN PAINT.
Thank you for your inquiry. The answer to your question is a high acid number is helpful for some formulations as it can provide improved adhesion over some substrates. It also depends on whether or not the alkyd is in an ambient cure paint formula with driers or a thermosett formula using an aminoplast crosslinker.
please, I want to write a proposal on the preparation of alkyd resins can anyone help with the procedures. Tnx
Sufficient fusion will ensure reduction of acid value with increasing viscosity
Soya fatty acid
Av 180
Iv 126
Alkyd resin process sample acid value is 11.5 but after thinning acid value is high 21 viscosity ok solid ok but acid value drop any process to give me please
Ogechukwu, contact me through [email protected]
May be during solvent addition, solvents vapors melted the phthalic anhydride from the condenser and mixed in the resin,that may increased the acid value
Hello!
Can anyone share with me the kinetic data for Alkyd resin plant for my fydp..
Thanks!
Email: [email protected]
Need to know on the reaction/roll of TPP(Tri-phenyl-phosphite) in the improvement of Color stability of Alkyds.
How to find R value in alkyd resin
Organic phosphites such as triphenyl phosphite stabilize polymers against oxidative degradation during higher temperature processing which takes place in alkyd polymerization as well as during use after synthesis is complete. Aryl phosphites such as triphenyl phosphite function as antioxidants by decomposing hydroperoxides to give phosphates and alcohols.
Could someone give me the formula of long oil Alkyd Tall oil base.
To reduce cycle time keep good reflux and slight increase amt of maleic anhydried. Up to 1 prcentage M A . After charging phthalic anhydried first one hour on reflux at 190 degree centi grade for reducing loss of phthalic
I’m working in a resin plant,iwant to know whether Soya bean fatty acids and Sunflower fatty acids when mixed, the outcome of the resin should be milkish or what?
Hello I am a PhD student working with alkyd resin, I was having issues cleaning my glassware and metal reactors, I was wondering if there was a standard cleaning procedure for alkyds?
first of all you will take caustic water boiling with 10% concentration for two hrs. then in heating condition discharge water, then you mannually clean your reactor. after that you will take solvent boiling.
This content is very helpful.
Hello I’m Nicolas and actually I’m working in a last year project about alkyd resins, I’m trying to find some information about the kinetics but I did not found anything about it. I want to know if you can help me with this information?
Thanks
Hi, Nicolas. Please contact me directly at [email protected].
Thank you,
Ron
Dip in ten to fifteen % concentration of NaOH Solution for one day
Could anyone kindly help me,that what percentage of malic anhydride can be added in rosinated and long oil alkyd resins.in what stage it has to be added.
Hi, Srinivas. Could you please contact Ron Lewarchik by using his website: https://chemicaldynamics.net/. Thank you!
What should be molecular weight of long oil alkyd
Dear Mr. Modi:
Please contact me at [email protected].
Thank you,
Ron
Hi every one,
is it ok to use adipic acid for alkyde?
Hi, Akbar.
Adipic acid is used in some alkyd formulations to improve flexibility depending on the level used as well as the other building blocks chosen in the alkyd.
Thanks for reading!
Ron
For your attention
I am working in a Soya long 60% resin manufacturing plant. BCT of Resin is 20 hour now. How can reduce BCT of Resin?
Good day!
Could you please provide a meaning for “BCT?”
Thank you,
Ron
Better way 196 av & iodin value 132
Abnormal batch handling point
1. A.v less viscosity is than required viscosity change pthalic anhydride processing the poly
2. A.v is more viscosity is high than required A.v change NPG/ glycerin for decreasing value
3.A.V lower side viscosity not getting under poly then decrease or stop nitrogen rate. down the water level from receiver slightly check controlling %NV for 30 min of .60 gm
4.during alkyd poly AV goes down fast but viscosity not pickuping fast during this decrease nitrogen rate ( AV goes down fast when reflux is strong.nitrogen rate fast gives extra pressure to vapour mass in reactor so, reflux strong. That’s why reflux gets slow when nitrogen rate slow
5. Viscosity is not increasing during poly down the water level in seperator. when we down the water level this space occupies by solvent. So batch solvent comes out and viscosity gets rise
Regards,
Gajanan
i am chemical engineer muhammad islam ,wrking as a process engineer in alkyd resin plant ,can some guide and help me chemical reaction of alkyd resin
Hi, Muhammad:
The answers you see are in this article: https://www.ulprospector.com/knowledge/3944/pc-basics-alkyd-resin-technology/
Thanks for reading,
Ron
hi sir please share the formulation of alkyd resin
Thank you for your inquiry. For additional specifics, there are several books available that provide additional detail and formulations of alkyd resins. One such book can be found on the following link: https://link.springer.com/chapter/10.1007%2F978-94-011-1220-8_5
Thank you,
Ron
Respected Sir, please guide that how to reduce the darkness of alkyd resin.
Dear Muhammad:
By darkness do you mean color or yellowness value?
Thank you,
Ron
Dear sir,
I am working on an alkyd resin plant and facing some problems regarding the color of resin.
The color of resin is dark brown but i want its color pale yellow. so please guide me to make resin color pale yellow.
Thanks
Dear Mr. Arslan,
A high color value in an alkyd can be caused from a number of issues including the absence of an antioxidant, high cook temperature, prolonged cook time or type of catalyst employed. Please contact me directly at [email protected] in the event that you desire additional consultation.
Thank you.
Pls I need your response on the following questions: 1 how to check if the product is alkyd resin 2 what will justify the quality of alkyd resin.
Dear Muhammad:
1. how to check if the product is alkyd resin – By the product do you mean a determination of the identity of a resin sample that you acquired? If so, the best way to determine the identity and type of resin sample you have is to perform FTIR spectral analysis. The use of FTIR coupled with spectral analysis software will identify the closest match of the resin to existing resins. The solvent should first be removed prior to performing the analysis.
2. what will justify the quality of alkyd resin. I’m not sure what you mean by justify the quality of the resin. The resin selection and quality of the resin is dependent on the intended use for the paint the resin will be used and the desired performance. Certain types of alkyds may be ideal for one application, but totally unfit for a different application.
Thanks for reading,
Ron
please I want to Know
1-what is the relation between the molecular weight of alkyd Resin and viscosity
2-also what is the relation between the molecular weight of alkyd Resin &the Drying Time of alkyd
3- How is the high solids paints have a lower molecular mass and show higher reactivity to autoxidation polymerization
I appreciate your effort
1. In general as the molecular weight increases for a given alkyd composition and solvent, the viscosity will increase as well. However the alkyd building blocks and structure also contributes to viscosity as does solvent selection. Normally the better the solvent for a given solvent borne alkyd, the lower the viscosity.
2. Dry time of an alkyd is dependent on multiple issues, one of the most important is the type of oil that is used in an alkyd. The higher the level of the conjugated unsaturation of the oil portion of the alkyd the faster the oxidative cure dry time. For example soya and linseed oil both have a very high level of conjugated unsaturation in the oil portion of the alkyd and will promote much faster oxidation cure than that of coconut oil which has a high level of saturated oil. Other factors are the oil content in the alkyd, as well as the alkyd structure and building blocks. For example a Vinyl Toluene alkyd will provide quicker dry to touch times due to the High Tg of VT.
3. There are many types of “lower molecular weight alkyds”, some of these types of alkyds such as chain-stopped alkyds use the addition of benzoic acid to react with hydroxyl and “stop the chain growth”. These resins as a class do not necessarily promote faster oxidative cure, but more so quicker dry to touch times due to presence of the formation of benzoate (to increase initial hardness). After full cure the ultimate solvent resistance of chain stopped alkyds is normally at best equivalent to higher molecular weight alkyds.
Thank you,
Ron Lewarchik
COULD YOU PLEASE INFORM US THE MATHEMATICALLY ADDITION % OF NCO TO ALKYD RESIN TO PRODUCE URETHENATED ALKYD RESIN.PLEASE ANSWER MY QUESTION ASAP
Please open the following link on the Prospector website for the calculation you are looking for. When reacting excess alcohol groups with isocyanate (normally TDI) you need to know the % hydroxyl in the alkyd and follow the equation listed after Table III. If the level of excess hydroxyl is high, this could result in a gel depending on the NCO:hydroxyl level you select.
Thank you for reading!
Dear sir,
I am working on an alkyd resin plant and facing some problems regarding the color of resin.
The color of resin is dark brown but i want its color pale yellow. so please guide me to make resin color pale yellow.
Thanks
Direct Reply
Mansoor,
Organic phosphites such as triphenyl phosphite stabilize polymers against oxidative degradation during higher temperature processing which takes place in alkyd polymerization as well as during use after synthesis is complete. Aryl phosphites such as triphenyl phosphite function as antioxidants by decomposing hydroperoxides to give phosphates and alcohols.