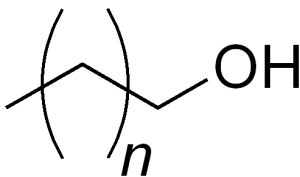 Fatty alcohols (EU) are an important class of materials used in numerous types of personal care products. Get familiar with their many uses, characteristics and product resources below.
Fatty alcohols (EU) are an important class of materials used in numerous types of personal care products. Get familiar with their many uses, characteristics and product resources below.
Typical uses include:
- Thickening anhydrous products like antiperspirant sticks
- Thickening oil in water emulsions via the formation of lamellar crystalline gel networks
- As emollients to modify skin feel
- As solvents (liquid branched type)
They are chemicals characterized as having a free primary, secondary, or tertiary hydroxyl group attached to a long chain group. Fatty alcohols are usually long chain primary alcohols, but they can also range from as few as 4-6 carbons to as many as 22-26 carbons. They can also be unsaturated and have methyl branching.
These chemicals occur widely in nature in plants/animals and normally are straight chain, even in carbon length. They are produced by transesterifying triglycerides (EU) with methanol followed by hydrogenating the resulting esters to the alcohol.
Fatty alcohols are also prepared synthetically. In the Ziegler process, ethylene is oligomerized using triethylaluminium followed by air oxidation. This process creates even-numbered alcohols. Alternatively, ethylene can be oligomerized to give mixtures of alkenes, which are subjected to hydroformylation. This process creates odd-numbered aldehydes, which are subsequently hydrogenated. For example, from 1-decene, hydroformylation gives the C11 alcohol.
In the Shell higher olefin process, the chain-length distribution in the initial mixture of alkene oligomers is adjusted so as to more closely match market demand. Shell does this using an intermediate metathesis reaction. The resultant mixture is fractionated and hydroformylated/hydrogenated in a subsequent step.
Guerbet alcohols are branched alcohols produced by the condensation of primary alcohols at temperatures of 180 – 300°C in the presence of alkaline condensing agents. They are normally liquid alcohols.
Recommended Suppliers
Natural based:
- Procter and Gamble – C8-18 alcohols under the CO designation.
- BASF (Cognis) – C12-22 alcohols under the Lanette trademark.
EU: BASF | Lanette - Sasol – C12-22 alcohols under the Nacol trademark.
EU: Sasol | Nacol
Synthetic straight chain:
Branched (Guerbet type)
Reference
Dr. Z Presents All about fatty alcohols (copyright 2000-Condea)
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Hi
I am working on a project on c8, c10 and c8-c10 fatty alcohols. Based on my study i found that these can be manufacture from natural source as well as synthetic sources.
Natural sources-Palm oil, Palm kernel oil, coconut oil
Synthetics sources- olefins and paraffins
Is my research correct ?
Can you please provide me the details manufacturing process with both the sources.
Any inputs from your end are very valuable.
Thank you
Interesting to have such information