Inert pigments absorb nearly no light, and therefore, by themselves in a cured paint film, do not stand out from a color perspective. Inert pigments have a refractive index similar to that of the vehicles used in paints, so they provide very little light-scattering. However, used in conjunction with opacifying pigments, they can provide enhanced opacity at lower cost. Inert pigments are also called fillers or extenders as they are normally lower in cost and occupy volume in the paint film. Other valuable functions they provide include improved mechanical properties, rheology adjustment, gloss adjustment, and enhanced barrier protection.
Critical Characteristics of Inert Pigments that Influence Paint Performance
- Mineralogy – Chemical composition, crystal structure, Hardness in Mohs (Fig. I)
- Physical Characterization – Brightness, refractive index, pH, inertness, oil absorption, purity and presence of soluble salts
- Particle Metrics – Particle size, shape, size distribution and aspect ratio
Looking for inert pigments for your coatings formulations?
Prospector has product listings for inert pigments. Get technical data, request samples and more now.
Search Inert Pigments
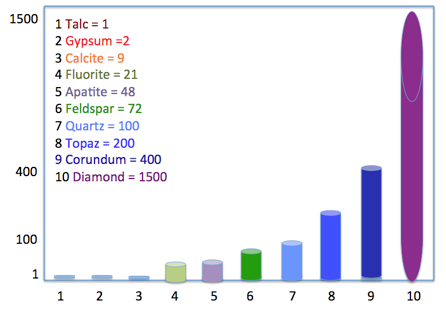
Per Figure I, talc would be a better filler pigment to improve sanding characteristics in a primer-surfacer, whereas a silica based pigment such as quartz (SiO2) would provide better scrub resistance in an interior architectural wall paint due to increased hardness.
The Chemical composition of a pigment can also play an enormous role in determining the overall impact on the performance. For example, calcium carbonate in exterior latex paint can degrade in the presence of acid rain, producing carbon dioxide and calcium bicarbonate, which is water soluble. This in turn causes the film to be porous and the calcium bicarbonate to migrate to the surface of the paint film, forming a light frosting of insoluble calcium carbonate.
Pigments that have a pH of less than 7 can exacerbate corrosion when used in metal primers. Aluminum in a pigment contributes to the acidity, whereas calcium, potassium, barium, and sodium provide alkalinity. If a pigment contains soluble salts, these salts can contribute to blistering when exposed to moisture.
Oil absorption is primarily a function of the pigment surface area, pigment chemistry and how easily it is wet by Linseed oil. Low oil absorption equates to lower vehicle demand and lower paint viscosity, especially in solvent born coatings. The refractive index (R.I.) of the pigment measures how light is bent when it passes from one medium to another. The greater the R.I., the more light is bent and the greater the opacity. Most filler pigments have an R.I. similar to that of the paint vehicle and therefore do not contribute to a high degree of opacity in the cured film. Some filler pigments, such as alkaline aluminum silicate (nephaline), have a fine particle size that contribute to TiO2 spacing and thus provide improved opacity at a lower cost.
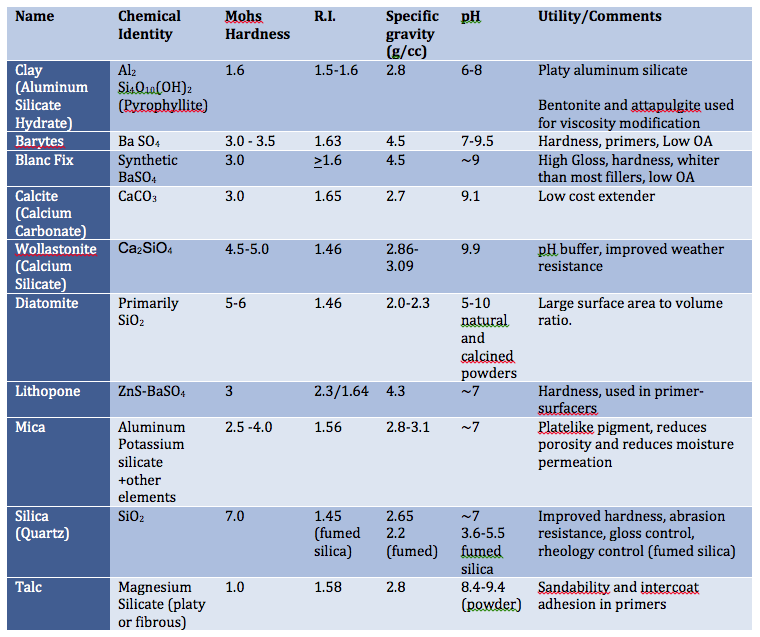
Table I lists a number of inert or filler pigments and some common characteristics. As pigment sources and manufacturers’ processes vary, it is best to reference the suppliers’ product data sheets for specific detail.
Particle Size, Size Distribution and Shape can play an important roll in determining what effect the inert pigment may have on paint performance. The following particles illustrated in Figure II may have the same “particle size” as indicated by a particle analyzer, but will provide different physical properties:
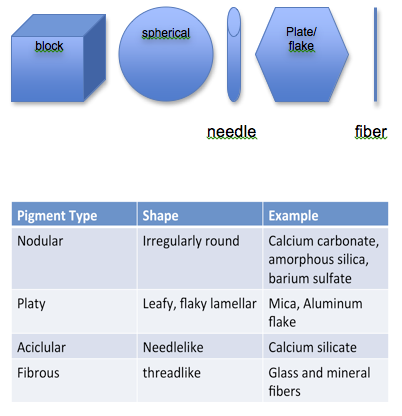
The aspect ratio in a needle or fiber particle is the ratio of mean length to mean diameter (a fibrous pigment 50 nm long and 5 nm wide will have an aspect ratio of 10). The aspect ratio in a plate-like particle is the ratio of the mean diameter of a circle of the same area as the face of the plate to the mean thickness of the plate. Needle or threadlike particles tend to enhance intercoat adhesion especially if they are oriented perpendicular to the surface, whereas platy pigments if oriented parallel to the surface can minimize oxygen and moisture permeation to the substrate. Examples of the latter include mica and micaceous iron oxide.
Median particle size can be misleading as, for example, two pigments may have the same median particle size, but vary dramatically in overall particle size distribution. The median particle size means that half the particles are larger and half are smaller. Particle size distribution is usually expressed as D10, D50, and D90. The subscript defines the percent of the total particles finer than the stated micron particle size.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Excellent article.Mr.Ron, clearly explaint the impact of fillers particle size and its structure.
N.SRIDHAR
OPERATIONS MANAGER
PRISMA PAINTS.
0097338417483
Very informative. Thanks for sharing.
Nice article, thanks to Mr. Ronald for this information. I deal with resins but interested in learning more about paints and pigments. Today I have learned something from you, thank you.
The particle size & shape of inert pigments has an effect on Colour , Gloss & other properties of a coating. So as a paint ,I have great appreciation for the topic
what about their oil absorption ? and colour ?