A polyurethane is any polymer made by the reactions between alcohols with two or more reactive hydroxyl (-OH) groups per molecule (diols, triols, polyols) and isocyanates that have more than one reactive isocyanate group (-NCO) per molecule (diisocyanates, polyisocyanates). Polyurethanes are in the class of compounds called reaction polymers, which includes epoxies, unsaturated polyesters, and phenolics. For example, a diisocyanate reacts with a diol:
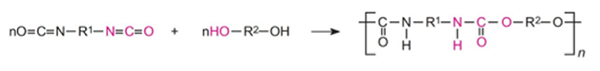
Polyurethanes are the most versatile of all the polymers and can be made liquid, rigid, pliable and stretchable, and can be spread, sprayed or molded. Because a variety of diisocyanates and a wide range of polyols can be used to produce polyurethane, a broad spectrum of materials can be produced to meet the needs for specific applications. The following are some of the many applications of polyurethanes:
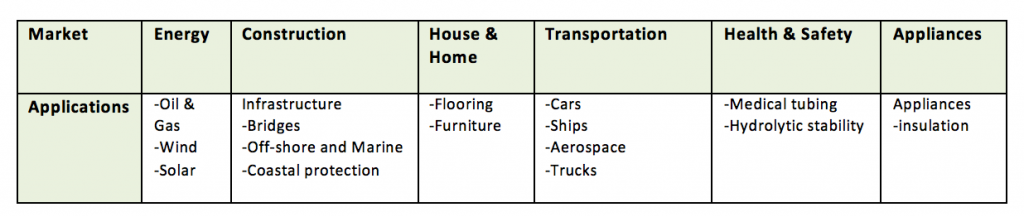
Of the many uses for polyurethanes, ~15% are used in Coatings, Adhesives, Sealants and Elastomers (CASE); however these are the segments of focus in this article.
Looking for polyurethane for your coatings formulations?
Prospector has more than 1800 search results for polyurethane products! View technical data and order samples now…
Search Polyurethanes
Chemistry – Isocyanates/Polyisocyanates
Of the roughly 85% of non-CASE applications, the polyisocyanates (PI) utilized are either Methylene Diphenyl Diisocyanate (MDI, CAS 101-68-8 and 9016-87-9) and Toluene Diisocyanate (TDI); 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). Both have come under intense scrutiny by the EPA within the past month, with continued concerns of worker safety and health with exposure to unreacted materials.
Both are aromatic compounds, and as such provide relatively poor resistance to ultraviolet (sun)light, and therefore are not used in coatings applications where sunlight is particularly harsh, but rather in uses where UV is not a concern. TDI is mainly used to make flexible polyurethane foam that can be found in a wide range of products, including furniture, bedding, carpet underlay and packaging. TDI is also used in the manufacture of some coatings, sealants, adhesives and elastomers.
TDI helps produce lighter automobile seating and headliners, saving weight and making vehicles more energy efficient. MDI is used primarily to make rigid polyurethane foams such as insulation for appliances such as refrigerators, and many other uses. Insulation made with MDI can help save heating and cooling costs, Vehicle parts like dashboards, steering wheels and bumpers are also made of MDI.
Less widely used, but still important, are the aliphatic diisocyanates, including hexamethylene diisocyanate (HDI), hydrogenated MDI (H12MDI), and isophorone diisocyanate (IPDI). However, even in coatings, only 3-5% utilize aliphatic PI, while the remaining 95-97% employ aromatics. HDI, H12MDI and IPDI are most often further reacted to form polyisocyanates, or pre-polymers, which are used to form color-stable polyurethane coatings and elastomers that can significantly enhance a product’s appearance, lengthen its lifespan and offer high abrasion resistance.
Covestro (a.k.a. Bayer Material Science) and Evonik are the main suppliers of H12MDI, while BASF and Covestro supply IPDI. There are several Asian companies that provide these as well.
Coatings prepared with aliphatic diisocyanates can have excellent resistance to abrasion, as well as superior weathering characteristics, including gloss retention and resistance to yellowing and chalking, as well as lengthening the time between painting cycles. Chemical-resistant coatings made with aliphatic diisocyanates help commercial airliners maintain the durability and resistance needed to withstand harsh atmospheric conditions.
Dependent upon the application and environmental considerations, polyurethanes can be solventborne or waterborne. Although great strides have been made in waterborne polyurethane chemistry, they typically do not perform as well as their solventborne analogs. A market need and emergent trend is to produce either a 1-part (1K) polyurethane or/and a 2-part (2K) that cross-links without the use of polyisocyanates. Dow Chemical has recently marketed Acrylic Polycarbamate technology that does not utilize PI.
Chemistry – Polyols
Polyols are polymers and have, on average, two or more hydroxyl groups per molecule. Polyether polyols are mostly made by co-polymerizing propylene oxide and ethylene oxide and with a suitable polyol precursor. Polyester polyols are made similarly to polyester polymers. The polyols used to make polyurethanes are not “pure” compounds since they are often mixtures of similar molecules with different molecular weights and mixtures of molecules that contain different numbers of hydroxyl groups, which is why the “average functionality” is often mentioned. Despite being mixtures, industrial grade polyols have their composition sufficiently well controlled to produce polyurethanes having consistent properties. It is the length of the polyol chain and the functionality that contribute much to the properties of the final polymer. Polyols used to make rigid polyurethanes have molecular weights in the hundreds, while those used to make flexible polyurethanes have molecular weights up to ten thousand or more.
Read part 2 now…
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Hello Mark, Do you know which grades Dow acrylic polycarbamates are and are they commercially available.
Gary,
Dow Chemical is constantly developing new products and improving on what is commercial. Since this was written 9 months ago, I suggest that you contact them directly. As it appears you are in the UK, I would suggest contact to the US although there is a European website as well:
http://coatings.dow.com/en
It is well-organized and you can get to what you need and to whom you should speak with an answer with a small effort.