 In 2013, the direct cost of corrosion was 3.1% of the 15.1 trillion in U.S. GDP, which in June 2013 is estimated to equal $500.7 billion. Corrosion is a an electrochemical process where the metal is oxidized by virtue of interaction with its environment, which results in the metal returning to its most stable oxidative state. This article will discuss those factors that influence corrosion, especially in regard to the use of coatings designed to protect the metal to which they’re applied. Accordingly, consideration of the fundamental factors that influence corrosion processes as it relates to the use of organic coatings will be considered herein.
In 2013, the direct cost of corrosion was 3.1% of the 15.1 trillion in U.S. GDP, which in June 2013 is estimated to equal $500.7 billion. Corrosion is a an electrochemical process where the metal is oxidized by virtue of interaction with its environment, which results in the metal returning to its most stable oxidative state. This article will discuss those factors that influence corrosion, especially in regard to the use of coatings designed to protect the metal to which they’re applied. Accordingly, consideration of the fundamental factors that influence corrosion processes as it relates to the use of organic coatings will be considered herein.
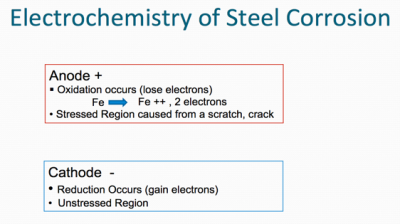
Metals desire to be in their most thermodynamically stable state, which, in simplified terms, is the naturally occurring state of matter in its lowest energy state. Metals ordinarily exist naturally as oxides (e.g. iron oxide, aluminum oxide, zinc oxide etc.), because oxides represent their lowest energy state. Oxidation occurs at the anode (positive electrode) and reduction occurs at the cathode (negative electrode). Corrosion is normally accelerated by the presence of water, oxygen, and salts (particularly of strong acids).
Looking for corrosion protection materials for your paint and coatings formulations?
Prospector has hundreds of listings for materials related to corrosion protection. View product data sheets, request samples and more now…
Search Anti-Corrosion Materials
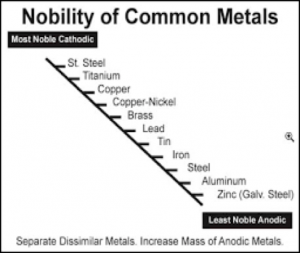
Figure I lists a series of metals and their ability to resist corrosion. The most common metals used in industry include steel (cold rolled and hot rolled steel), aluminum, galvanized steel (hot dip and electrogalvanized steel) as well as galvalume. The latter two metal substrates utilize either a zinc layer or an aluminum/zinc layer respectively on the surface of the steel to enhance corrosion resistance.
Even though aluminum and zinc are less noble than steel, when not coated with an organic coating, they provide longer-term improved corrosion resistance than steel. When steel rusts, the corrosion product (ferric oxide) is loosely attached to the surface, whereas in the case of aluminum or a zinc/aluminum alloy, the corrosion products form a more tightly knit adherent layer to the metal surface that decreases the subsequent rate of corrosion (Table III).
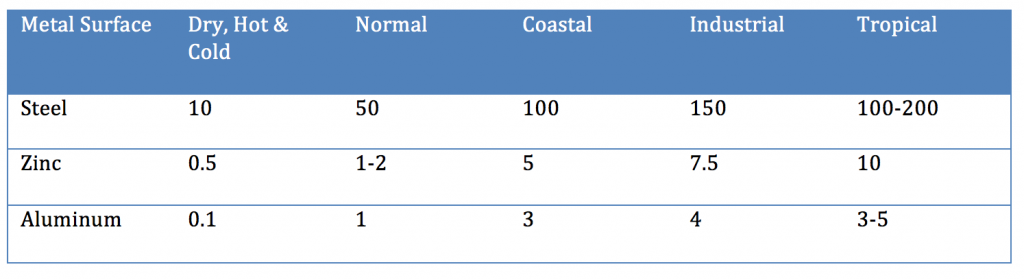
Multiple factors influence the performance of organic coatings over an oxidizable substrate. First and foremost is the initial and wet adhesion when the coated substrate is exposed to the environment in which it is expected to perform. If a coating does not offer excellent adhesion and ultimately wet adhesion, the coating will fail at the metal interface. Additional considerations are the metal type (e.g. steel, aluminum, galvanized etc.) and the pretreatment and cleanliness of the surface. If the metal surface is not properly cleaned and prepared, the coating will lack adequate adhesion and premature failure will result.
Organic coatings are semipermeable membranes and their ability to provide a barrier to the penetration of plays an important role in providing protection to the metal surface. Environmental factors that influence the rate of corrosion include moisture, pH of the moisture, wet and dry cycles, soluble salts, temperature and time. For example, moisture, soluble salts, higher temperatures and longer exposure times all normally exacerbate corrosion of coated metal. Film thickness plays a vital roll in deterring the penetration of moisture, salts and oxygen as thicker films deter the ingress of the environment.
Pigment selection, PVC and the use of corrosion inhibitive pigments all impact corrosion resistance, film permeability, and corrosion passivation. The proper choice of corrosion inhibitive pigments can help ameliorate the rate of corrosion through cathodic and/or anodic inhibition. Cathodic inhibition disrupts the flow of electrons at the cathode (-) through the supply of electrons from a less noble metal (e.g. zinc as in a zinc rich coating), or a suitable corrosion inhibitive pigment that is present in the coating. Anodic inhibition disrupts the flow of electrons at the anode (+) for example by anions (e.g. PO4 anion). Please see the July 3, 2014 Prospector article for additional information on corrosion inhibitive pigments, as well as the August 15, 2014 Prospector article that details the effect of pigment volume concentration on coatings properties.
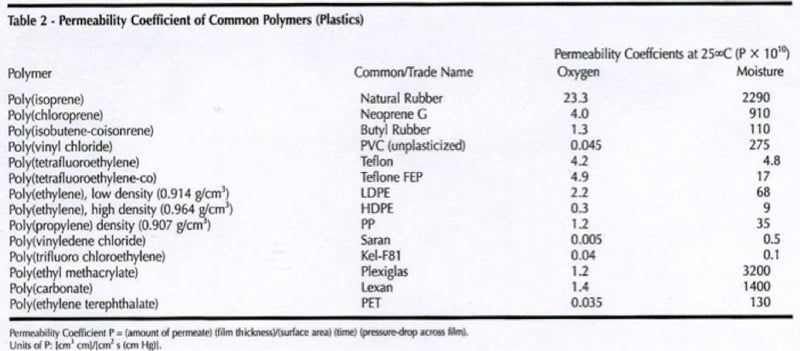
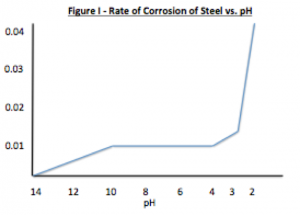 Figure I displays the effect of pH on the rate of corrosion. The rate of corrosion is constant between pH of 4 – 10 and greatly accelerates at a pH lower than 4 and decreases above a pH of 10. The byproducts of metal corrosion are normally basic, accordingly the selection of a resin that provides saponification resistance is an important consideration along with the selection of a resin/coating system that provides wet adhesion and minimizes the penetration of moisture and oxygen. For example most alkyds are prone to saponification as the ester linkages hydrolyze. As resin Tg and cross-link density increase, moisture and oxygen penetration decrease. In addition, low permeability rates help to provide wet adhesion as less water will desorb when the coating is removed from it’s service environment. Resins with a high amount of aromatic character (bisphenol A based epoxies, polycarbonate and styrenated resins) have low oxygen permeability. Many halogenated resins such as poly (vinyl chloride), chlorinated rubber and fluorinated polymers such as poly (vinylidene fluoride) all have low water solubility and thus low moisture permeability rates (Table II).
Figure I displays the effect of pH on the rate of corrosion. The rate of corrosion is constant between pH of 4 – 10 and greatly accelerates at a pH lower than 4 and decreases above a pH of 10. The byproducts of metal corrosion are normally basic, accordingly the selection of a resin that provides saponification resistance is an important consideration along with the selection of a resin/coating system that provides wet adhesion and minimizes the penetration of moisture and oxygen. For example most alkyds are prone to saponification as the ester linkages hydrolyze. As resin Tg and cross-link density increase, moisture and oxygen penetration decrease. In addition, low permeability rates help to provide wet adhesion as less water will desorb when the coating is removed from it’s service environment. Resins with a high amount of aromatic character (bisphenol A based epoxies, polycarbonate and styrenated resins) have low oxygen permeability. Many halogenated resins such as poly (vinyl chloride), chlorinated rubber and fluorinated polymers such as poly (vinylidene fluoride) all have low water solubility and thus low moisture permeability rates (Table II).
In summary, the formulation of coatings to deter corrosion is a complex undertaking and depends on the metal substrate, resin selection, service environment, and the pigment level and type. For additional information concerning resin and material selection to formulate corrosion inhibitive coatings, please navigate to www.ulprospector.com.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Leave a Reply or Comment