Introduction
Flexibility is an important mechanical property of coatings. Insufficient flexibility can have several causes, and it can create a range of problems. This article focuses on the most common causes and possible consequences of internal stress. Also, some methods to prevent stress in coatings are discussed.
Film formation
During film formation, a liquid paint transforms into a solid coating. Two processes that can take place during film formation play a crucial role in many systems.
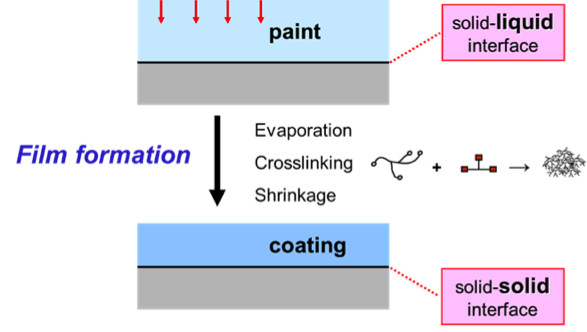
First, evaporation of volatile components, like organic solvents or water, can occur. A film shrinks when material leaves the system because of evaporation. Secondly, a chemical reaction of resin and crosslinker, called crosslinking, takes place in many systems. Preferably, shrinkage only occurs in the direction perpendicular to the paint-air interface.
Causes of stress
Several phenomena can result in stress building up in the coating. First, the adjustment of evaporation and crosslinking might not be optimum. Consider a system in which crosslinking is fast and evaporation is slow. In such a system, a gel is formed when there is still a considerable amount of solvent present in the film. When evaporation continues, the voids that appear when solvent molecules leave the film, will be filled with the binder system. Stress can build up when the binder system has limited mobility caused by the high crosslink density.
In solvent-free, UV-curing systems, the binder matrix contracts because of reaction shrinkage. Even though the system does hardly loose material because of evaporation, stress can build up in the binder matrix during film formation.
A low amount of binder material is present in systems that have a high loading of solid particles. The demands, with respect to flexibility, of the binder system goes up when the percentage of solid particles increases.
Stress can evolve in a coating when the dimensions of the substrate change because of fluctuations in temperature and/or humidity or under the influence of mechanical force.
In general, it can be said that internal stress will build up when relaxation of the binder system is insufficient to cope with the changes that take place in the system.
Possible problems caused by stress
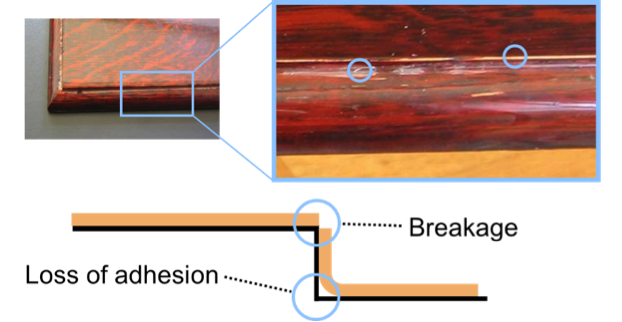
A variety of undesired phenomena can take place when internal stress builds up during film formation. Cracking of the coating occurs when the strength of the film is insufficient to cope with the stress.

A coating can break at sharp edges of the substrate. Also, the coating can be pulled loose from the substrate. This loss of adhesion is often called delamination.
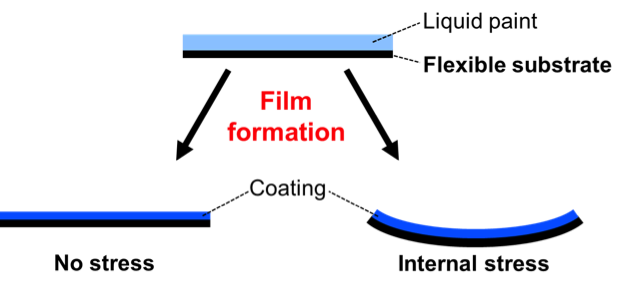
Determination of stress
It is often difficult to determine whether or not stress builds up in a coating. In a simple test, a system is applied onto a flexible inert substrate. The system will curl when stress builds up during film formation.
In most coatings, internal stress expresses itself via film defects as mentioned above: cracking, breakage and/or loss of adhesion.
Stress prevention
The most important attention points, with respect to the prevention of stress, are the binder system and the quality of film formation. In systems in which both evaporation and crosslinking takes place, the two processes must be adjusted to each other. Stress in systems in which reaction shrinkage occurs can be prevented by using reactive flexibilisers in the system. Finally, in systems with high particle loading, stress can be reduced by using a more flexible binder system or by optimising film formation.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Leave a Reply or Comment