A hydrophobic definition means “tending to repel or fail to mix with water.” Coatings that offer a hydrophobic (EU) or superhydrophobic surface can impart multiple advantages to the coating surface and substrate they are applied to. Advantages may include decreased dirt retention, self-cleanability, improved moisture and corrosion resistance, as well as extended life expectancy of the coating and substrate. To fully explain and quantify hydrophobicity, it is necessary to define the relationship between contact angle and the hydrophobic/hydrophilic (EU) character of a surface.
Figure 1 – Contact Angle for Hydrophobic Coating Surface and a Hydrophilic Coating Surface
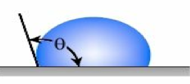
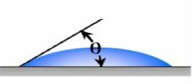
Figure 2 – Contact Angle and Superhydrophobicity
Accordingly, the surface characteristics can create different coatings, ranging from hydrophilic (water-loving) coatings to superhydrophobic coatings, which are highly water-repellent. Several factors impact the contact angle of a water drop on the surface of a coating. These include the macro, micro, nano-surface profile, and the surface tension of the coating on which the water droplet is resting. Surface tension is the elastic tendency of liquids that make them acquire the least surface area possible.
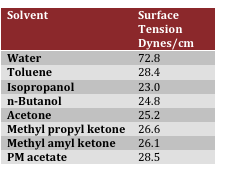
As Table 1 below illustrates, water has a higher surface tension than common solvents used in the paint industry. This is attributed to the high attraction of water molecules for each other as a result of hydrogen bonding. Another important factor in determining the hydrophobicity of coatings is the microscopic geometry of the surface.
Table 1 – Surface Tension of Paint
Nature has multiple examples of superhydrophobic and hydrophobic definition. One of the most notable surfaces is that of the Lotus leaf. The contact angle of water on the surface of a Lotus leaf is greater than 150°. The cause of self-cleaning properties of the Lotus leaf is the hydrophobic water-repellent double structure of the surface. This enables the contact area and the adhesion force between surface and droplet to be significantly reduced and results in a self-cleaning process allowing water to readily roll off the leaf and collect dust deposits on the way. This micron size double structure is formed at the surface of the plant and it’s comprised of needle-like projections from the surface that are covered by wax.

The wax-covered projections are 10 to 20 µm in height and 10 to 15 µm in width. These waxes are hydrophobic and form the top layer of the double structure. Some plants show contact angles up to 160° and are called super-hydrophobic, meaning that only 2–3% of the surface of a water droplet is in contact with the surface. Since the surface contact area is less than 0.6%, this leads to the self cleaning effect.
Thus far, we’ve defined the factors that contribute to the hydrophobicity or the lack thereof including contact angle, surface structure, and why most organic solvents tend to wet a surface better than water as a consequence of their lower surface tension. The next segment will concentrate on how to impart greater hydrophobicity to a coating system, especially from a surface perspective.
To maximize the surface hydrophobicity of a coating, the surface energy (EU) should be as low as possible. A low surface energy, coupled with an appropriately structured surface, maximizes hydrophobicity. Surface energy has the same units as surface tension (force per unit length or dynes/cm). A high surface tension liquid such as water will have maximum hydrophobicity and thus have poor wetting (high contact angle) over a coating surface that has a low surface energy. As Table II illustrates, surface energy can vary greatly depending on the nature of the surface that comes in contact with water.
Table II – Surface Energy of Materials[1]
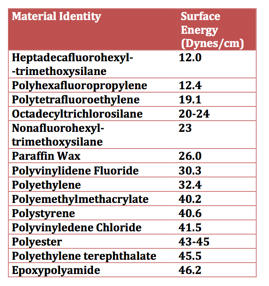
For example, a coating comprised of polyhexafluoropropylene (12.0 Dynes/cm) on the surface will provide a more hydrophobic surface than that of polymethylmethacrylate (EU) (40.2 Dynes/cm). In general terms, to provide the greatest hydrophobicity, the material’s most hydrophobic moiety should be positioned on the surface. For example, if an organofunctional trimethoxysilane (EU) is used for surface modification, the methoxysilane (EU) groups should be engineered to be positioned at the surface. Perfluoro and aliphatic (EU) groups at the coating surface offer greater hydrophobicity than that of ester or alcohol groups. For example, from lowest to highest surface tension:

Providing increased hydrophobicity throughout a properly engineered coating can also provide additional attributes such as improved corrosion and moisture resistance.
Accordingly, resin selection, flattener, extender pigments and opacifier (EU) pigments can also be selected to maximize hydrophobicity. Secondly, formulations utilizing nanoparticles (EU) must be tailored to provide proper acceptance rather than as a drop to achieve a desired property. In summary, properly formulated coatings utilizing nanoparticle technology can achieve performance attributes heretofore not obtainable by other means. Some suppliers of materials to enhance surface hydrophobicity listed in Prospector include BYK, Evonik Industries Ag Functional Silanes, ICM, Momentive, Phibro, Shin-Etsu Silicones of America, Tianjin Boyuan New Materials Co., Ltd., Evonik Industries AG Silica and Wacker.
Suppliers available in Europe: BYK, Evonik Industries Ag Functional Silanes | Momentive | Tianjin Boyuan New Materials Co., Ltd. | Evonik Industries AG Silica | Wacker
For additional information concerning the selection of materials to enhance hydrophobicity, please navigate to www.ulprospector.com (EU).
[1] Gelest 2006 Product Brochure
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Amazing effect! Nice article dear Ron, thank you for publishing.
Excellent Mr.Ronald.The points are so crisp and deep in subject.Good article.Gives good clarity and idea.
N.SRIDHAR
Which p0lymer do you suggest for high hardness and highgl0ss hydroph0bic coating that cures via m0isture ?
thank you for your helpful article.
I have some questions here and i hope that you would answer them for me.
I’m a student and I need some information for my next presentation.
1/ Can glue or tape stick on a hydrophobic surface?
2/ Is hydrophobic paint enviromentaly friendly?
3/ would hydrophobic surface be destroy by heat or radiation?
4/ How long would hydrophobic paint last in nature?
Very nice article.“superhydrophobicity / Lotus effect”
Can I find beginner and advance level formulation for the mesonary pavers coating.
With best Regards
Hello Pana, following are the answers to your questions:
1/ Can glue or tape stick on a hydrophobic surface? It depends on the type of hydrophobic surface. The compositions of these surfaces can vary quite dramatically. Some hydrophobic surfaces are comprised will accept a solventt borne glue, watrborne glues can be more challenging. Tapes vary in composition and adhesive strengths as well and a such adhesion is difficult to predict, however tapes with high adhesive strengths are more likely to have good adhesion.
2/ Is hydrophobic paint enviromentaly friendly? Environmentally friendly is broad term and can mean a variety of things such as low Volatile Organic Content (low VOC), or be derived from renewable materials. The simple answer is that the characteristics of hydrophobicity and “environmentally friendly” are mutually exclusive.
3/ would hydrophobic surface be destroy by heat or radiation? Some Hydrophobic surfaces are not heat stable (lotus leaf) and some are (teflon). Again it is a function of the materials used.
4/ How long would hydrophobic paint last in nature? A hydrophobic coating is very light stable that provides good hydrolytic stability will provide greater longevity.
Normally these compositions are proprietary to the manufacturer. Sometimes suppliers of resins provide formulations for select applications such as this. You can locate some sample formulations on Prospector.
Sir,
Very nice article. Thanks for publishing.
Could you please explain the effect of hydrophobic materials on asphalt pavement?
Hello Aiswarya,
Thank you for your question. Do you mean the application of a hydrophobic coating to the surface of asphalt or modification of the asphalt to be more hydrophobic or other?
Ron Lewarchik
Application of hydrophobic coating to the surface of asphalt pavement.
Could you explain more about hydrophobic coating to aplly in alluminium cookwares?
The answer to your question has several facets to it. Please let me know if you require a consultation in this regard for a more thorough response.
What are the ingredients should be used in what radio
The exact ratio and identity of the ingredients is proprietary. If you are interested in developing a formulation for a specific application I suggest that you contact the suppliers of siloxane and silane ingredients that can be found by doing a search in the Prospector web site.
Useful post! I really need this type of article.. this is very useful for me.
Thank you for all your help. Your service was excellent and very FAST. Many thanks for you kind and efficient service. I have already and will definitely continue to recommend your services to others in the future.
Thank you for your article. I have some questions about this issue.
Is there any chance to supply hydrophobic character to a polymer composite structure, by adding hydrophobic agents/ ingredients directly to the resin instead of topcoat coating?
Hi Emre:
I have some questions for you:
• Yes you can add hydrophobic materials to the resin, but do you mean to react the materials into the resin or just to blend or disperse the materials into the resin? In either case the answer is yes, but the means to achieve the end result is very different.
• Please describe what you mean by a polymer composite structure…..is this to obtain layers of coatings or layer(s) of a polymer or polymers composed of resin with hydrophobic modification (reacted in or otherwise)?
• Please explain “instead of topcoat coating” in the context of your question?
Thank you,
Ron