 Introduction
Introduction
Intumescent paints are designed to temporarily protect objects from losing their strength in a fire.1,2 The objective is to increase the time for evacuation of humans and animals and for the fire brigade to arrive. Steel, for example, loses its strength at temperatures above 500°C. Therefore, an intumescent paint applied on a steel structure must arrange that the temperature of the steel stays below 500°C for as long as possible.
An intumescent coating expands in a fire, thus forming a thick protective foam layer called char. The char insulates the object from the fire: the foam forms a barrier between the fire and the object. Also, the chemical reactions that take place during expansion are endothermic, implying that these reactions absorb heat. The object and the intumescent layer are cooled because of the endothermic reactions.
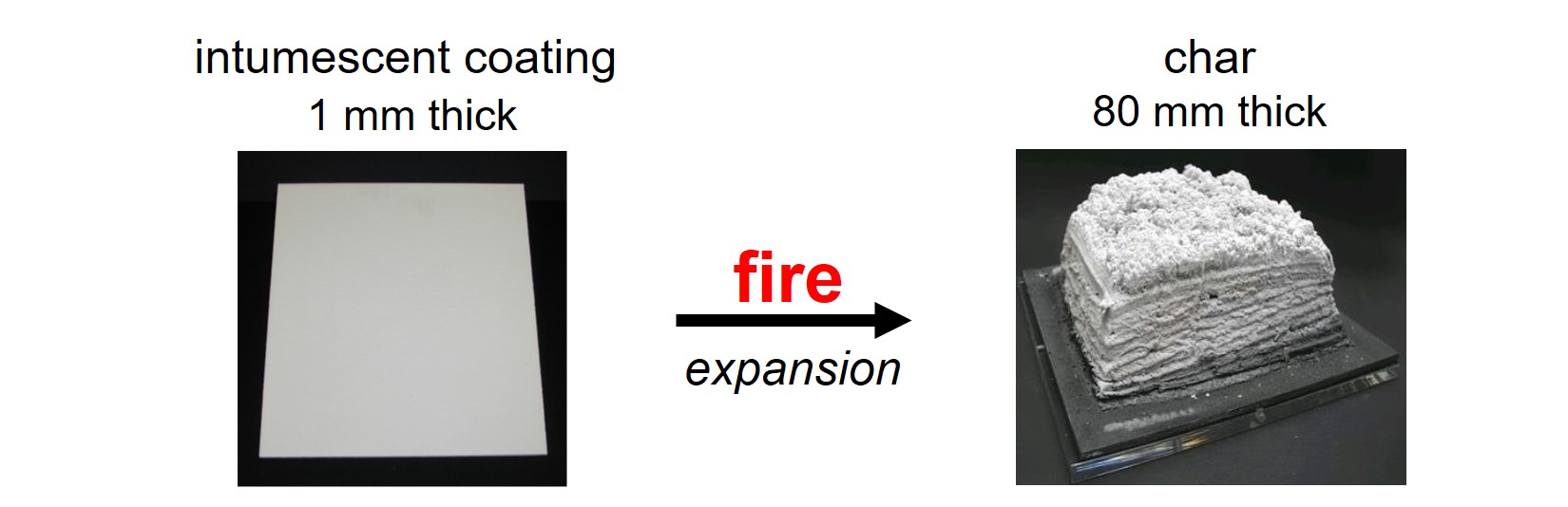
Several concepts, binder systems and additives are used for intumescent paints. This article is about the combination of four raw materials that are most often used in intumescent paints: ammonium polyphosphate, pentaerythritol, melamine and titanium dioxide.
Ammonium polyphosphate
APP (ammonium polyphosphate) is a white crystalline solid that decomposes at high temperatures, giving ammonia gas (NH3) and polyphosphoric acid (HO-[PO3H]n-H).
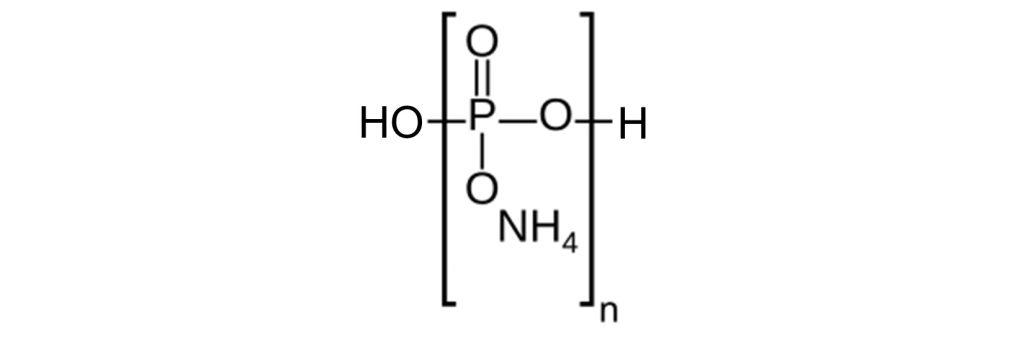
Two categories of APP are used in intumescent paints. Phase-II (long-chain) APP, with n > 1,000, has a low water solubility. Phase-II APP starts to decompose at around 300°C. Phase I APP (n < 100) is partly soluble in water. Phase-I (short-chain) APP starts to decompose at around 150°C.
Clariant supplies a range of APP products for intumescent paints under the tradename Exolit® AP.
Melamine
Melamine is a white crystalline solid that starts to decompose at around 340°C, forming NH3, CO2 and H2O.
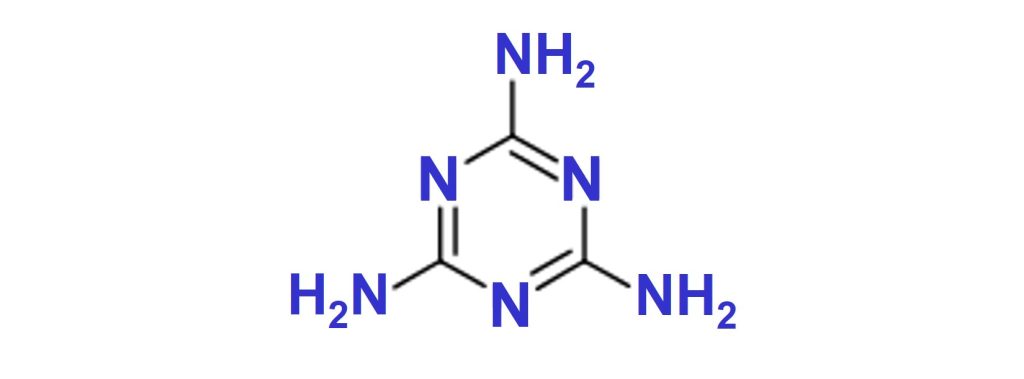
Melamine for intumescent paints is supplied by OCI under the tradename Melafine®.
Pentaerythritol
Pentaerythritol is a crystalline white solid. Before melting, pentaerythritol starts to decompose at around 190 °C, forming aldehydes.
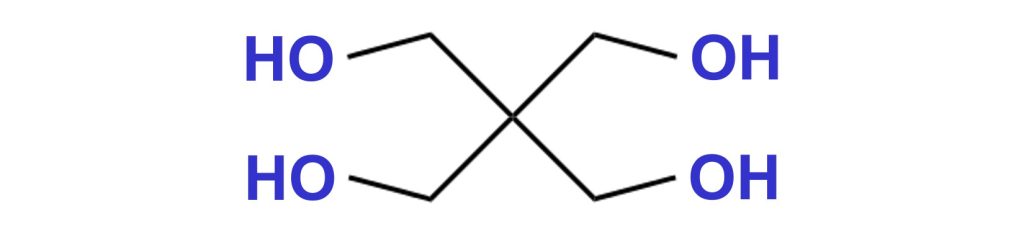
Perstorp supplies different variations of mono- and di-pentaerythritol under the tradename Charmor™.
Titanium dioxide
Titanium dioxide (TiO2) is a crystalline white solid. Rutile TiO2, having a melting point of around 1,800°C, is used in paints as white pigment to give hiding power and white color. In an intumescent coating in a fire, TiO2 builds an inorganic structure with polyphosphate that is released from APP. There is a wide variety of rutile TiO2 pigments that can be used for intumescent paints.
How does it work?
An intumescent coating on an object must stay intact when the temperature rises in the first phase of a fire. For this reason, the key ingredients are crystalline. These raw materials melt at temperatures substantially higher than 100°C. At a temperature of around 200°C, chemical reactions start to take place within the intumescent coating. Gasses (like NH3, CO2 and H2O) are formed in these chemical reactions, thus giving foam formation. At the same time, a structure must be formed that is strong enough to withstand the fire for a long time and stabilize the gases in a foam-like structure called char. The intumescent layer acts as a barrier, giving thermal insulation of the object against the heat of the fire. The formation of gases is an endothermic process. This implies that heat is required for the gasses to form. Because of this, the chemical reactions deliver a cooling effect.
The experts do not agree on what chemical reactions exactly take place during expansion. Especially the role of pentaerythritol is unclear. The general view is that pentaerythritol reacts as a polyalcohol with polyphosphoric acid, thus forming an organic/inorganic ester structure. Zybina and Gravit3 believe that pentaerythritol decomposes, thus forming aldehydes, followed by a crosslink reaction between melamine and those aldehydes, giving organic melamine-aldehyde resins.
Ultimately, when the fire keeps on going, and the temperature rise of the intumescent layer continues, all carbon (C) and nitrogen (N) that are present in the layer are oxidized, and an insulating white foam structure of titanium phosphates remains.
References
- Article Fire Retardant Coatings: Answers to Your Burning Questions, Wally Kesler, 13 July 2018.
- Article Fire Retardants/Fire Resistant/Intumescent, Marc Hirsch, 23 February 2022.
- Book Intumescent Coatings for Fire Protection of Building Structures and Materials, Olga Zybina & Marina Gravit, 2020.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.


