 Things transform from a regulatory standpoint and chemistry, especially when you talk about bio-based products or the types of things you need to disperse in a system. Over time, there has been less use of solvent-based paints and an increased use of carbon black and new organic pigments that have required up to 200% dispersant solids on pigment. The dispersants are generally high molecular weight and high viscosity.
Things transform from a regulatory standpoint and chemistry, especially when you talk about bio-based products or the types of things you need to disperse in a system. Over time, there has been less use of solvent-based paints and an increased use of carbon black and new organic pigments that have required up to 200% dispersant solids on pigment. The dispersants are generally high molecular weight and high viscosity.
Making a broad statement, there were only sodium, potassium, and ammonium dispersants for many years. There were some other ones that serve the purpose for specific applications, such as pigment slurries or even soya lecithin for colorants. But the change in pigments for many applications necessitated a like change in dispersants. It wasn’t enough to change the molecular weight of dispersants. Equipment was created to disperse materials faster in the laboratory, which led to scale up to production.
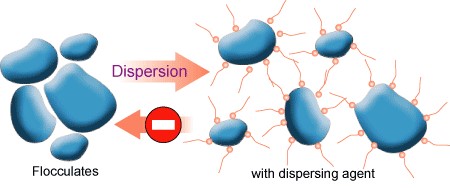
A dispersant is an additive that increases the permanency of a suspension of pigments in a liquid medium. The pigment-dispersing step is the most challenging and time/energy consuming part of the paint manufacturing process. This is generally because of the difference in surface energy between the solids (pigments and extenders) and liquids (polymers and solvents. The grinding of the agglomerates are dissociated into a dispersion of particles. In the process, dispersants (surfactants) are used that mainly prevent reassociation of the pigment and extender particles. The dispersants are adsorbed onto the particles and hinder close approach of particles by charge repulsion effects (ionic dispersants) or by steric effects (nonionic dispersants).
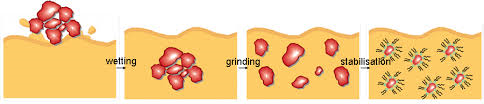
Dispersing and wetting agents are utilized to stabilize pigment dispersions. They often use steric hindrance to avoid flocculation of the pigment particles. Since a dispersant is used to help the suspension of fine particles of a solid in a liquid phase, it must be able to totally wet the solid particle and eliminate any and all air between the pigment and liquid medium. Dispersants act by being absorbed on the surface of the pigment particles with the other end of the molecule exposed and usually charged. Like charges repel, the independently coated pigment particles repel each other and stay uniformly dispersed.
Because the individual pigments are all so chemically different – some are attracted to water, and some are not – the dispersants have to be chemically different. Pigment dispersants are necessary to improve the wetting of the pigment and enhance its suspension. The choice of dispersant depends on the nature of the pigments and if the coating is a waterborne or solvent-borne system. Dispersants are offered for organic and inorganic pigment systems and for both solvent- and waterborne systems. Pigment dispersion is one of the most important, critical and complex steps in paint manufacture.
In the wetting stage, the liquid in the mill displaces air from the pigment surfaces. Shear force, applied by a mill, is necessary to break down pigment agglomerates. As pigment particles become adequately dispersed, dispersants that are now on the surface of the pigment particles prevent reagglomeration of the pigment.
Because of their hydrophobic nature, organic pigments are challenging to disperse in aqueous media. Surface-active compounds that are amphoteric in nature are usually effective for both organic and inorganic pigments that are particularly hydrophobic.
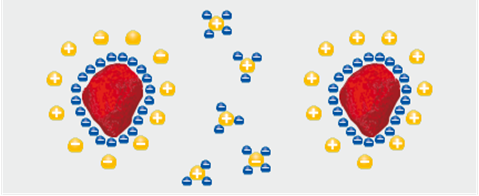
In aqueous systems, dispersants must disperse the pigment and form an affiliation between the pigment and the dispersing medium that is water. This is especially true with carbonaceous materials such as carbon nanotubes. There are ionic water-loving (hydrophilic) and oil-loving (lipophilic) types available on the market. The hydrophobic part of the molecule is attracted to the pigment particle and surrounds the particle with an ionic layer. Pigment separation by mutual repulsion takes place. These cannot be relied on for in-can stability and need to be used then with nonionic surfactants. Ionic, anionic and amphoteric surfactants are also essential for good dispersion, and contribute to the latex paint system by helping to optimize hiding power, flow, and leveling.
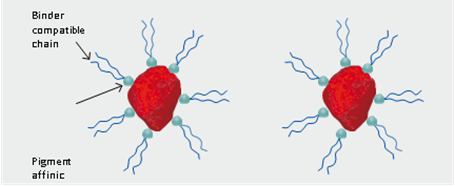
Pigments are usually easier to disperse in solvent-borne resin systems; however, higher solids systems will sometimes show pigment flocculation problems. This can be the result of the lower-molecular-weight polymers or oligomers having less of a buffering effect on the pigments and thereby permitting closer pigment-to-pigment contact. These are zero-VOC and alkyl phenol-free to allow reformulation of existing platforms to comply with new requirements. Amphoteric dispersants have been developed that are effective for preventing flocculation of carbon black and other organic and inorganic pigments in higher-solids systems. These dispersants have also been able to keep viscosity from acetylenic diols are also sometimes added. Another widely used class of dispersants is based on soya lecithin. These have been used for many years as pigment wetting agents, grinding aids and dispersion stabilizers in oleoresinous formulas. This class of agent is also available in water-soluble or water-dispersible forms. These are used in much the same applications in latex formulations. The water-compatible versions are also very useful in formulating universal colorants and as agents in both water and solvent formulas to improve universal colorant compatibility and color development. Typically, pigments are used to modify the optical properties of a cured film by introducing decorative color, enhancing opacity or controlling gloss. In addition to promoting visual effects, these materials are also commonly used as rheology modifiers, extenders and corrosion inhibitors. However, in all cases, the key to efficiently promoting the desired functionality rests with the ability to control both the distribution and particle size of the added pigment(s) within a coating formulation.
The first factor is, of course, the additive’s influence upon wetting and dispersing properties; the second factor is the summation of the additive’s influence upon all the properties within the formulated coating system.
Other resources:
http://www.inkline.gr/inkjet/newtech/tech/dispersion/
Pigment Dispersants Yield Colorful, High-Performance Results – Lubrizol
High-Performance Dispersants for Automotive Paints | Where business meets science (basf.com)
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



I remember the good old days using Morpholine Soya Fatty Acid in the “60’s.
Excellent article.
How can you differentiate a sedimentation caused by flocculation from a settling caused by gravity?
If you were to look at the particles with a microscope, you would see flocculation (groups of particles stuck together) if they do flocculate, and individual particles if it was settling by gravity or not settling in all.