
First commercialized by Bayer (Germany) and General Electric (USA) in the 1950s, polycarbonate (PC) is a widely used family of thermoplastic polymers. Polycarbonate is a copolymer in that it is composed of several different monomer types in combination with one another.
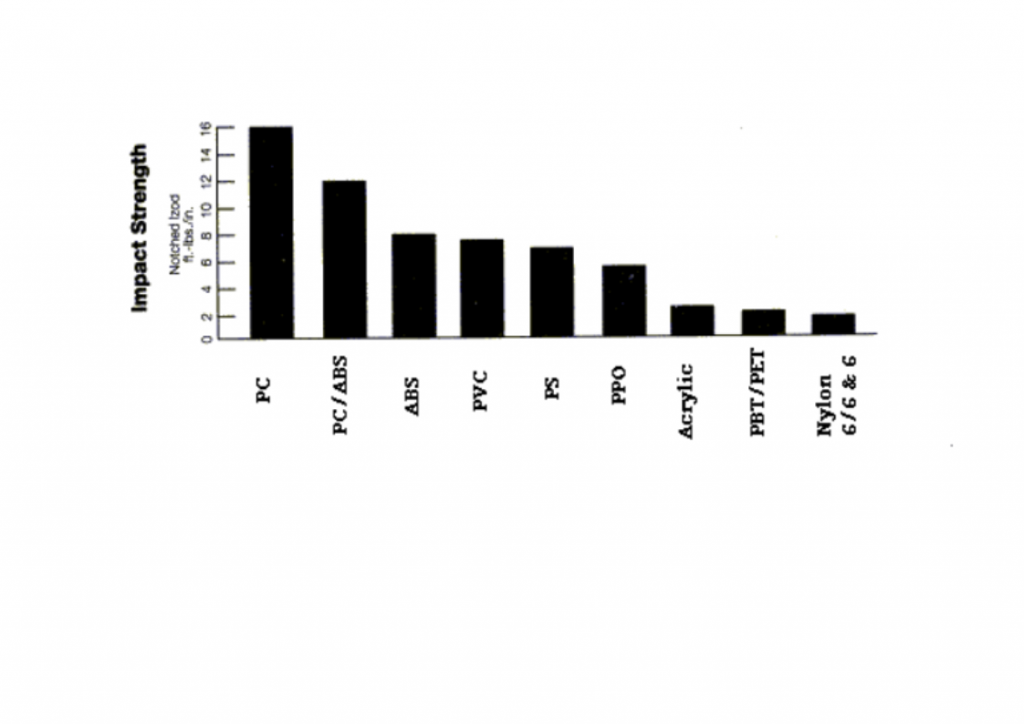
The grades of polycarbonate are noted for strength and particularly toughness. The family has the highest impact resistance of any thermoplastic, outstanding dimensional and thermal stability, exceptional machineability, stain resistance and is non-toxic with low water absorption. The high impact strength makes it resistant to repeated blows, shattering and spalling.
Some grades are optically transparent, with the optical properties of special grades transparent up to 32mm thick, comparable to those of the other popular clear plastic, polymethyl methacrylate (PMMA, acrylic). In fact, polycarbonate is highly transparent to visible light, with better light transmission than many kinds of glass. Polycarbonate is around 250 x stronger than glass and 30 x stronger than acrylic.
Limitations
Unfortunately, although it has high impact-resistance, it has low scratch-resistance, which means that a hard coating has to be applied to polycarbonate sheet, eyewear lenses and exterior automotive parts made from polycarbonate.
Other limitations include:
- Low fatigue endurance
- Mechanical properties degrade after prolonged exposure to water at over 60°C
- Attacked by hydrocarbons and alkalis
- Proper drying before processing needed
- Yellows after long exposure to UV
- Poor creep resistance (can be improved with the addition of glass or carbon fiber reinforcement)
Temperature behavior
In addition to being stronger than acrylic, polycarbonate will hold up longer to extreme temperatures. Polycarbonate is an amorphous material, meaning that it does not exhibit the ordered characteristics of crystalline solids. Typically, amorphous plastics demonstrate a tendency to gradually soften – they have a wider range between their glass transition temperature and their melting point – rather than exhibit a sharp transition from solid to liquid, as is the case in crystalline polymers.
Polycarbonate maintains its properties over a wide range of temperatures, from -20 to 140°C. Its glass transition temperature is around 147°C, and it softens gradually above this point and flows above about 155°C.
Standard polycarbonate resins are not suitable for long term exposure to UV radiation and may embrittle. The primary resin can have UV stabilizers added.
Processing
Polycarbonates are easily worked, molded, and thermoformed. Common methods used to produce polycarbonate parts include:
- Extrusion
- Injection molding
- Blow molding
- Thermoforming
- 3D Printing
To make strain-free and stress-free products, tools must be held at high temperatures, generally above 80°C. That said, low molecular mass grades are easier to mold than higher grades, but their strength is lower as a result. The toughest grades have the highest molecular mass, but they are much more difficult to process.
Polycarbonate can be deformed plastically without cracking or breaking. Unlike most plastics, it can be processed and formed at room temperature using sheet metal techniques and heating may not be necessary. This makes it valuable in prototyping applications where transparent or electrically non-conductive parts are needed, which cannot be made from sheet metal. Acrylic is very brittle, so cannot be used in this way.
Chemistry

The main polycarbonate material is produced by the reaction of bisphenol A (BPA) and phosgene COCl. Some products made from polycarbonate contain the precursor monomer BPA; this has made polycarbonate containers for food storage controversial. An alternative route to polycarbonates entails transesterification from BPA and diphenyl carbonate.
Applications
Electronic components, glazing, CDs/DVDs, optical reflectors, bottles, glasses, food containers, protective eyewear, display screens, mobile phone housings, bullet-proof “glass”. Tinted PC is used for the purposes of reducing glare, for example, to cover lighted signs on the road, to protect LEDs and to reduce glare. Special grades can be sterilized and may be used for medical applications.
Alloys and Blends
Polycarbonate blends provide a balance between various properties, performance and productivity.
PC/Polyester Blends: suitable where high chemical resistance is required. PC/PBT blends offer higher chemical resistance than PC/PET blends due to PBT’s higher crystalline behavior, whereas PET blended grades offer superior heat resistance.
PC/ABS Blends: the toughness and high heat resistance of polycarbonate combine with the ductility and processability of ABS.
Recycling
Researchers are also working to develop new processes for recycling polycarbonates into another type of plastic—one that does not release (BPA) into the environment when it is used or dumped into a landfill.
Many companies have developed bio-based polycarbonates but there are certain limitations regarding the cost of production.
They include:
- DURABIO by Mitsubishi Chemical Corporation
- POLYSORB Isosorbide by Roquette
- LEXAN PC resin based on Certified Renewable Feedstock by SABIC
Recently, a breakthrough has been made at the Korea Research Institute of Chemical Technology (KRICT) where researchers have created a bio-polycarbonate made largely from glucose. Unlike earlier biopolymers, the team claims that this new bio-polycarbonate has the strength and durability to match its petrochemical counterpart, paving the way for commercialization.
Find materials using more than 600 material property filters with Prospector’s Plus Plan. Upgrade today to take advantage of the advanced tools and engineering data!
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.



Thanks for the article. A few questions: why is polycarbonate susceptible to scratch? Also, when you talk about PC/ABS blend, what’s the difference between toughness and ductility?
Scratch resistance: not an easy question to answer! The scratch resistance of plastics is multi-faceted in nature, from base polymer selection, through additive technologies, to testing methods. Certain polymers – like crystalline polypropylene (PP) scratch easily, while others – like amorphous Acrylonitrile Butadiene Styrene (ABS) – have hard surfaces that resist scratching. But polycarbonate is amorphous, as are all trnsparent polymers! It is definitely related to surface hardness, and the hardness of acrylic is greater than that of polycarbonate.
Also, filler additives such as minerals, silicones, or nanoclays will affect scratch and mar surface visibility. The overall goal is to continually match surface appearance durability to the expected end product lifecycle.
Toughness and ductility: the difference is subtle. Ductility is the ability of a material to deform easily, that is to undergo plastic deformation without failure – for example, to be stretched, crushed or bent.
Ductility also contributes to another material property called toughness. Toughness combines strength and ductility in a single measurable property and requires a balance of both. It is the ability of a material, not only to plastically deform without fracturing, but to absorb energy as well. Technically it is the energy required to deform a material to failure (typically defined by the integrated area under a stress-strain curve).
Fracture toughness is a property that is indicative of a material’s resistance to fracture when a crack (or other stress-concentrating defect) is present.
Thanks, a good overview.
Is there a better technology for making FR PC than using halogenated materials. I understood to make UL V-0 or 5V a salt technology can also work
Does polycarbonate have any failure susceptibility to alcohol? Particularly isopropyl-acohol?
One of the limitations mentioned is poor creep resistance. Is this relative to semi-crystalline materials or other amorphous materials?
Hello,
Thanks for this overview article.
I have a question: Will heating polycarbonate to higher temperatures (around 275 to 350°C) will have any effects on its chemical composition? Thanks
All polymers are predominantly organic in nature and decompose around 350 to 700 deg C. It would depend on how long the exposure was for – a bit like the human body! Most polymers would lose much of their intrinsic strength and stiffness at those sorts of temperature. Polycarbonate has a glass transition temperature of about 147 °C (297 °F), so it softens gradually above this point and flows above about 155 °C (311 °F).
Thermoset materials like phenolics (Bakelite) have high temperature resistance, they are used as ablatives in exhaust nozzles of rockets, because when they degrade at high temperatures they form a residue having excellent heat insulating properties, hence preventing heat conduction into the other parts of the rocket/shuttle.
Some thermoplastic polymers (like the fluoropolymers and the polyimides and polysulfones) might just stretch to 300C, but at the expense of cost and other properties.
Most applications at these temperatures would be outside the range of most polymers. Designers would look to metals or ceramics or MMCs (metal-matrix composites).
GLARE (Glass laminate aluminum reinforced epoxy) is a relatively new composite, very low density though operating temperature around 600C. It is more of a replacement for aluminum than for polymers and is three to 10 times ore expensive than aluminum sheet.
What is your application?